Cupric chloride (II) chelate compounds by using 1-pyridyl-beta-carboline as ligand, and synthesis method and application thereof
A synthetic method and compound technology, applied in the field of medicine, can solve the problems of production cost limitation, drug resistance and side effects, in-depth research, etc., and achieve good medicinal value and strong anti-tumor activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Embodiment 1: the compound shown in formula (II) is the synthesis of 1-pyridine-beta-carboline (KL)
[0047] 1) Dissolve 1.60g (10mmol) of tryptamine in 70mL of dichloromethane, then add 1.07g (10mmol) of pyridine-2-carbaldehyde, and heat to reflux for 8 hours; after the reaction, distill under reduced pressure to obtain the crude compound 1 ;
[0048] 2) Dissolve 2.49g (10mmol) of compound 1 in 80mL of glacial acetic acid, then add 13.4g of manganese acetate hydrate (Mn(Ac) 3 ·nH 2 (0), heated to 80°C, reacted overnight, evaporated the solvent, added 100mL water, extracted 3 times with ethyl acetate, combined the organic phases, evaporated the solvent to obtain an oily crude product, and then purified by flash liquid chromatography (V 石油醚 :V 二氯甲烷 =1:1), to obtain light yellow crystal compound 2 (yield about 73%).
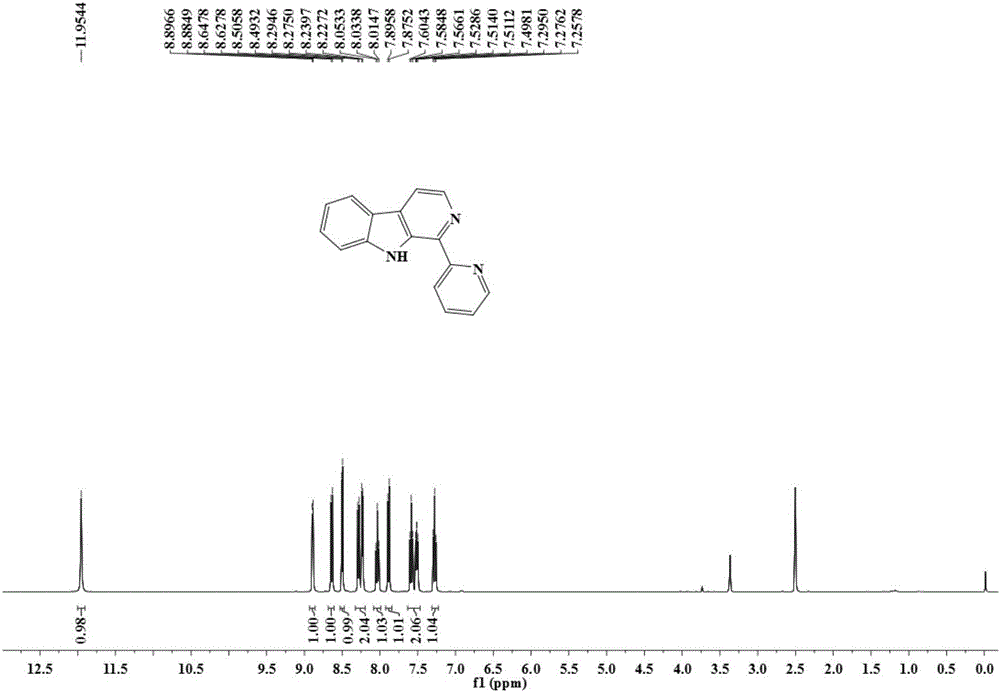
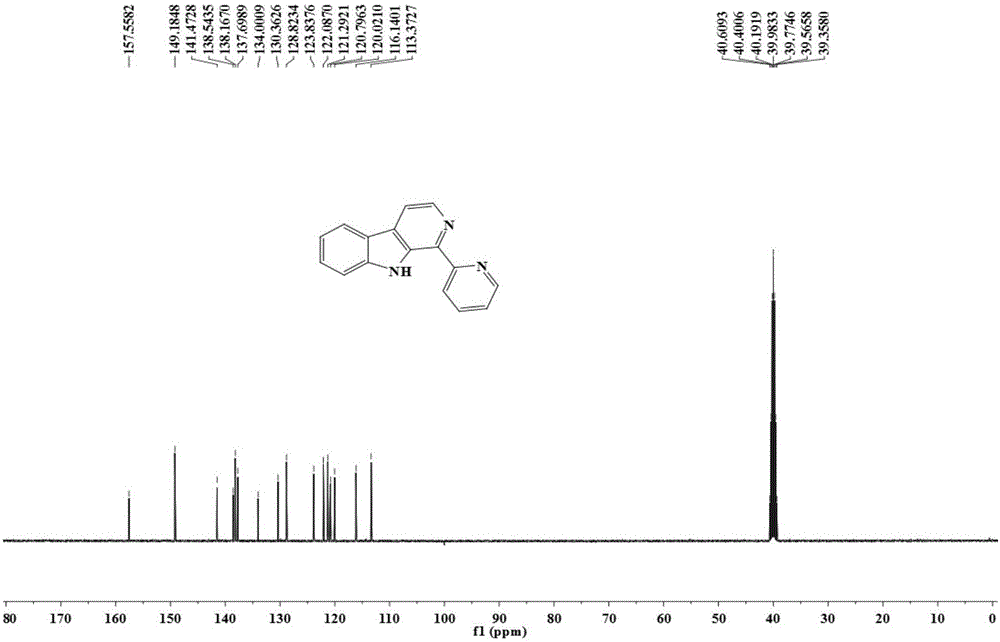
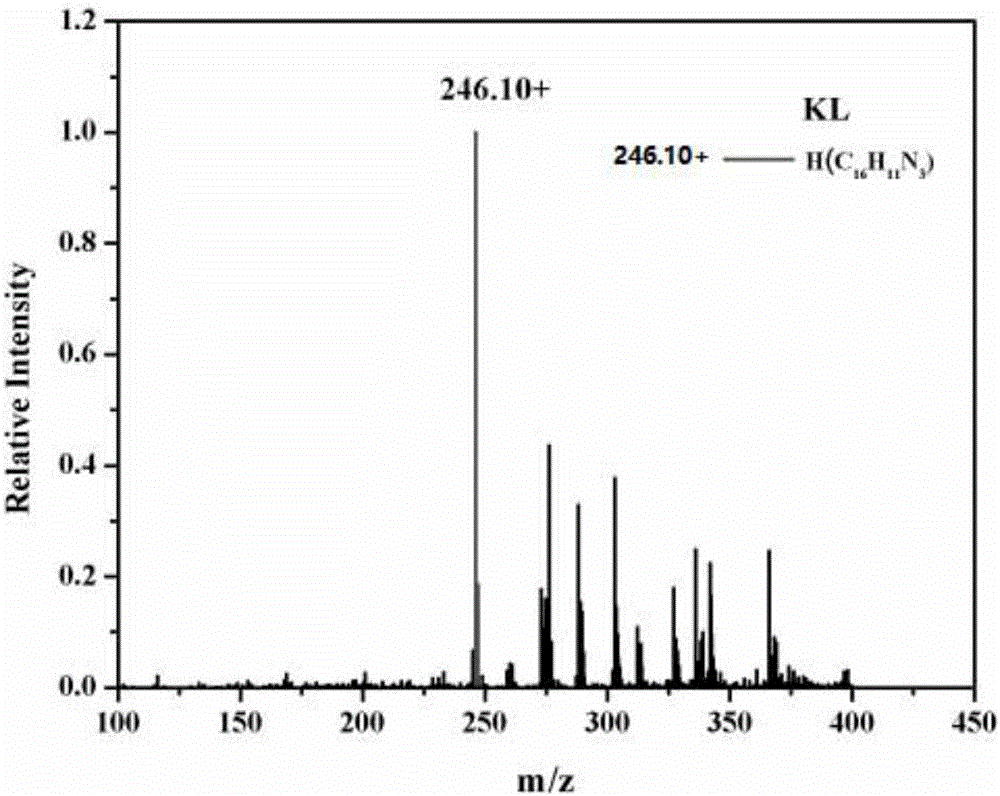
[0049] The resulting pale yellow crystals were subjected to proton nuclear magnetic resonance spectrum, carbon nuclear magnetic resonance spectrum, elec...
Embodiment 2
[0057] Embodiment 2: the synthesis of ligand KL
[0058] 1) Dissolve 1.60 g (10 mmol) of tryptamine in 70 mL of toluene, then add 1.07 g (10 mmol) of pyridine-2-carbaldehyde, and heat to reflux for 6 hours. After the reaction, distill under reduced pressure to obtain the crude compound 1;
[0059] 2) Dissolve 2.49g (10mmol) of compound 1 in 80mL of glacial acetic acid, then add 13.4g of manganese acetate hydrate (Mn(Ac) 3 ·nH 2 (0), heated to 70°C, reacted overnight, evaporated the solvent, added 100mL water, extracted 3 times with ethyl acetate, combined the organic phases, evaporated the solvent to obtain an oily crude product, and then purified by flash liquid chromatography (V 石油醚 :V 二氯甲烷 =2:3), to obtain light yellow crystal compound 2 (the yield is about 55%).
[0060] The obtained pale yellow crystals were analyzed by H NMR, C NMR, electrospray mass spectrometry and single crystal diffraction, and it was determined to be the target product 1-pyridine-β-carboline.
Embodiment 3
[0061] Embodiment 3: the synthesis of ligand KL
[0062] 1) 1.60g (10mmol) tryptamine is dissolved in the mixed solvent that is made up of the methanol of 30mL and the ethanol of 40mL, then add 1.07g (10mmol) pyridine-2-carboxaldehyde, heat to reflux for 12 hours, after the reaction finishes, reduce Press distillation to obtain crude product compound 1;
[0063] 2) Dissolve 2.49g (10mmol) of compound 1 in 80mL of glacial acetic acid, then add 20mmol of Pb(Ac) 4 , heated to 90°C, reacted overnight, evaporated the solvent, added 100mL of water, extracted 3 times with ethyl acetate, combined the organic phases, evaporated the solvent to obtain an oily crude product, and then purified by flash liquid chromatography (V 石油醚 :V 二氯甲烷 =3:2), the light yellow crystal compound 2 was obtained (the yield was about 67%).
[0064] The obtained pale yellow crystals were analyzed by H NMR, C NMR, electrospray mass spectrometry and single crystal diffraction, and it was determined to be the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com