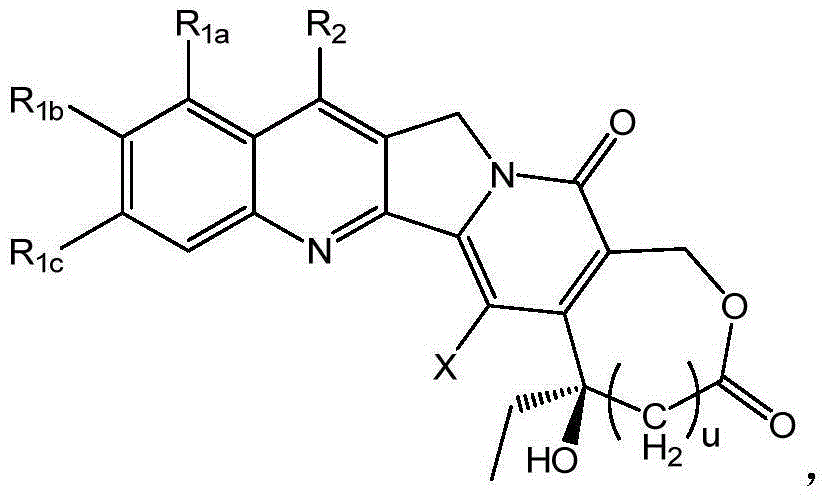
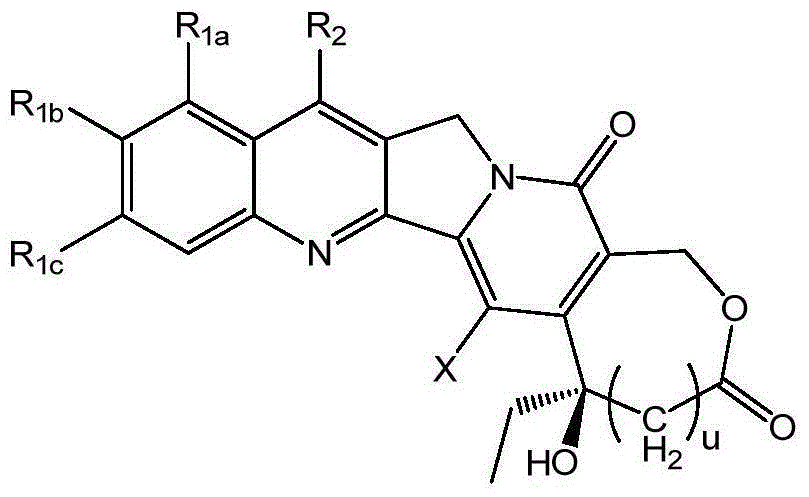
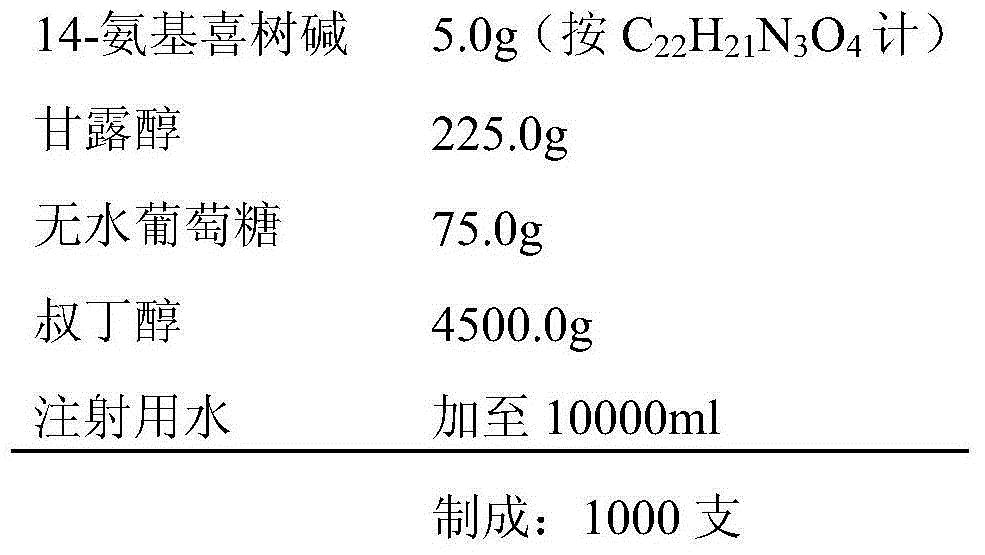
Injection preparation containing camptothecin analogue, and injection, preparation and application thereof
A technology of injection preparations and derivatives, applied in the field of medicine, can solve the problems of reduced solubility, efficiency affecting the implementation effect, and low solubility of active ingredients
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0115] Solubility
[0116] Take excess active ingredients, add 10ml of solubilizing excipients as a solvent, seal, and stir in a constant temperature water bath at 30°C, protected from light, at 150r / min for 24 hours. The dissolved amount is shown in the table below.
[0117] Effect of Solubilizing Excipients on Solubility
[0118]
[0119] It can be seen that the amount of drug dissolution is closely related to the type and concentration of co-solvent or surfactant. The solubilization effect of high concentration of co-solvent or surfactant is obvious, but the amount of drug dissolved is also significantly reduced after the concentration of excipients is reduced. At the conventional application concentration of the preparation, the solubilization effects of surfactants Tween 80, HS15, Cre EL and latent solvents propylene glycol and PEG400 were relatively good, while the dissolution effects of other excipients were poor.
[0120] Study on the Freeze-drying Effect of tert-...
Embodiment 2
[0132] Solid Fraction: Screening of Excipients 1
[0133]
[0134] Screening of Excipients 2
[0135]
[0136] Reconstitution method: The solvent used for reconstitution is an aqueous solution (both w / v) containing HS15 (7%), propylene glycol (10%), and ethanol (5%), and each bottle (containing 5 mg of active ingredient) is injected into the powder for injection 10ml of solvent, shake for about 2min.
[0137] Reconstitution result:
[0138]
[0139]
[0140] It can be seen that according to prescription 1, the moldability of the drug alone by freeze-drying is poor, and the reconstitution of the powder is poor.
[0141] According to prescriptions 2-10, they are all better than prescription 1, and they all meet the requirements when used within the specified time, especially prescription 6, which has good drug formability, low product impurity, good reconstitution effect, and better comprehensive indicators such as moisture and stability. Ease of use.
[0142] In...
Embodiment 3
[0151] Embodiment 3 liquid part: special solvent prescription
[0152] The prescription is composed as follows (%, w / v):
[0153]
[0154] Each bottle (containing 5 mg of active ingredient) of powder for injection is injected with a specified volume of special solvent, and shaken for about 2 minutes.
[0155]
[0156]
[0157] All the formulas can quickly reconstitute the lyophilized powder, and the amount of impurities in the reconstituted solution does not change much within 4 hours, indicating that the chemical stability of the reconstituted solution is good.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com