A dalfampridine slow-release tablet and a preparing method thereof
A technology of dafampridine and sustained-release tablets, applied in the directions of pharmaceutical formulation, coating, pill delivery, etc., can solve problems such as reducing production cost and strengthening labor protection, and achieves the effect of reducing production cost, improving labor protection, and simple prescription
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
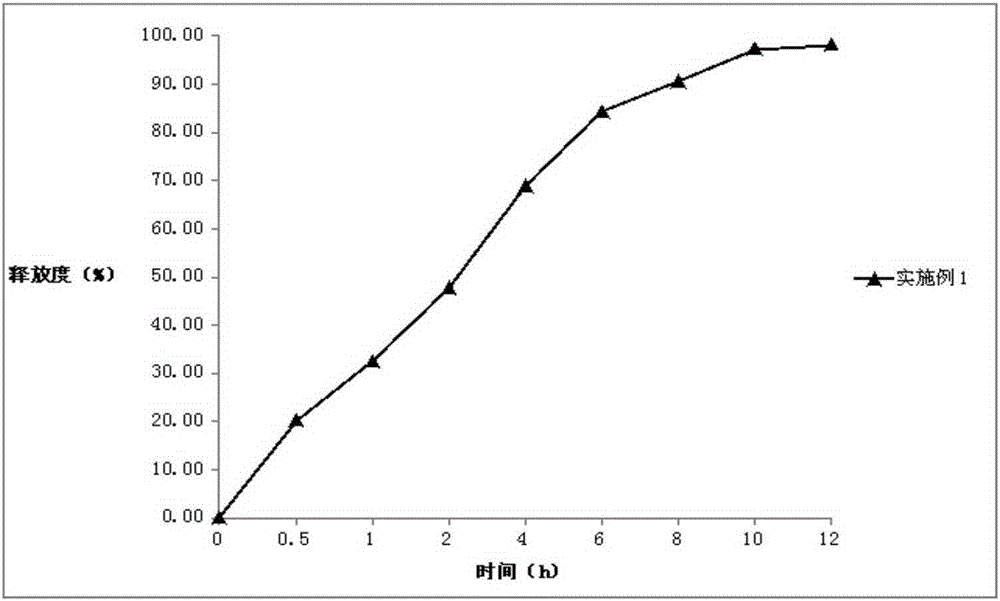
[0044] Embodiment 1: Darfangyridine Sustained-release Tablets of the present invention
[0045] prescription:
[0046]
[0047]Preparation:
[0048] a), in the isolator, dissolve the prescribed amount of darfanpyridine and hydroxypropyl methylcellulose E5Lv in 50% ethanol aqueous solution, and use it as a drug-containing adhesive for subsequent use;
[0049] b), hydroxypropyl methylcellulose K100Lv and pregelatinized starch are placed in a fluidized bed, mixed uniformly, the drug-containing binder is sprayed into the fluidized bed at a constant speed for granulation, and dried;
[0050] c), after drying, granulate, add colloidal silicon dioxide and magnesium stearate, mix, compress into tablets, and wrap into film coating.
Embodiment 2
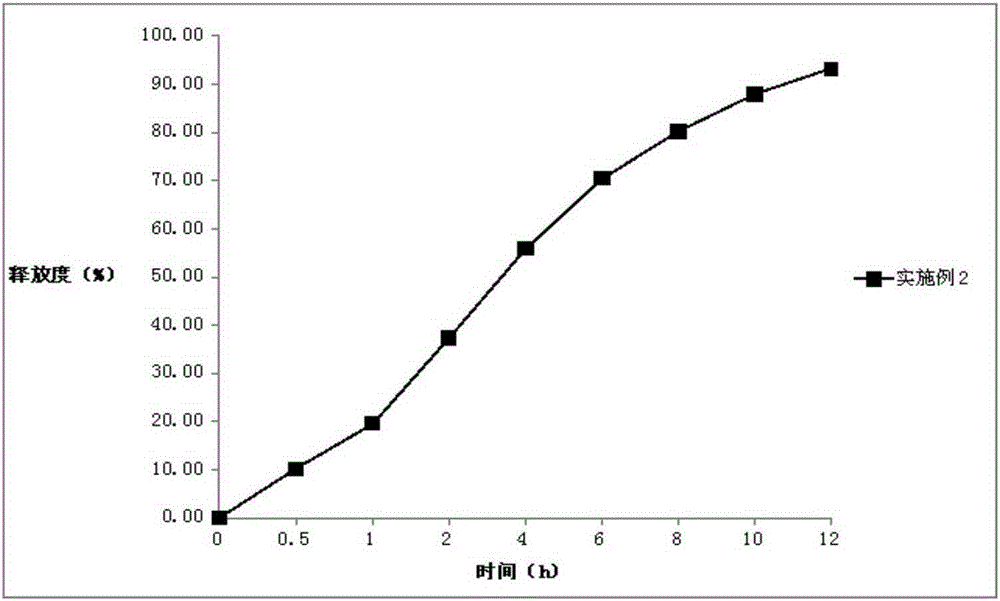
[0051] Embodiment 2: Darfangyridine Sustained-release Tablets of the present invention
[0052] prescription:
[0053]
[0054] Preparation:
[0055] a), in the fume hood, the prescription amount of darfanpyridine and povidone are dissolved in 40% ethanol aqueous solution, and they are used as the drug-containing adhesive for subsequent use;
[0056] b) Hypromellose, microcrystalline cellulose, and lactose are placed in a wet granulator, mixed evenly, and the drug-containing binder is sprayed into the high-shear wet granulator at a constant speed for granulation, Fluidized bed drying;
[0057] c), after drying, granulate, add colloidal silicon dioxide and magnesium stearate, mix, compress into tablets, and wrap into film coating.
Embodiment 3
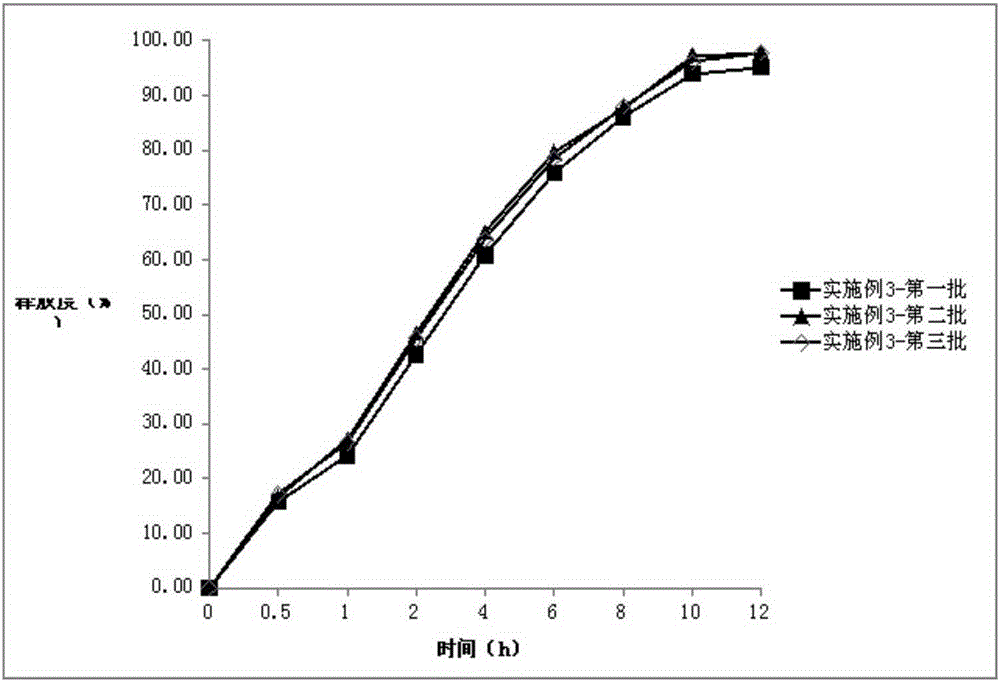
[0058] Embodiment 3: Darfangyridine Sustained-release Tablets of the present invention
[0059] prescription:
[0060]
[0061]
[0062] Preparation:
[0063] a), in the fume hood, dissolve the prescribed amount of darfanpyridine and hydroxypropyl cellulose in 70% ethanol aqueous solution, and use it as a drug-containing adhesive for subsequent use;
[0064] b) Put hypromellose and microcrystalline cellulose in a wet granulator, mix well, spray the drug-containing binder into the high-shear wet granulator at a constant speed for granulation, and fluidize bed dry,
[0065] c), after drying, granulate, add colloidal silicon dioxide and magnesium stearate, mix, compress into tablets, and wrap into film coating.
[0066] The preparation of three batches was repeated, and three batches of dalfampridine sustained-release tablets were prepared respectively.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com