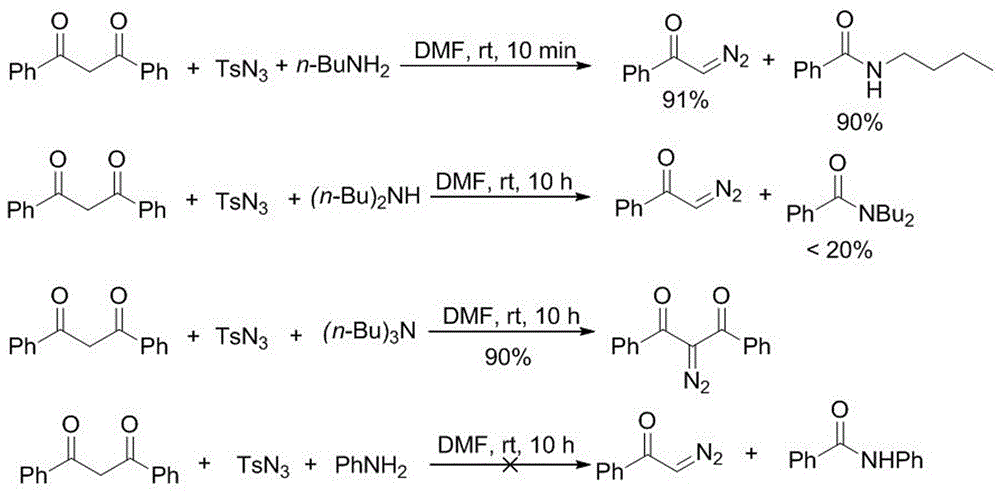
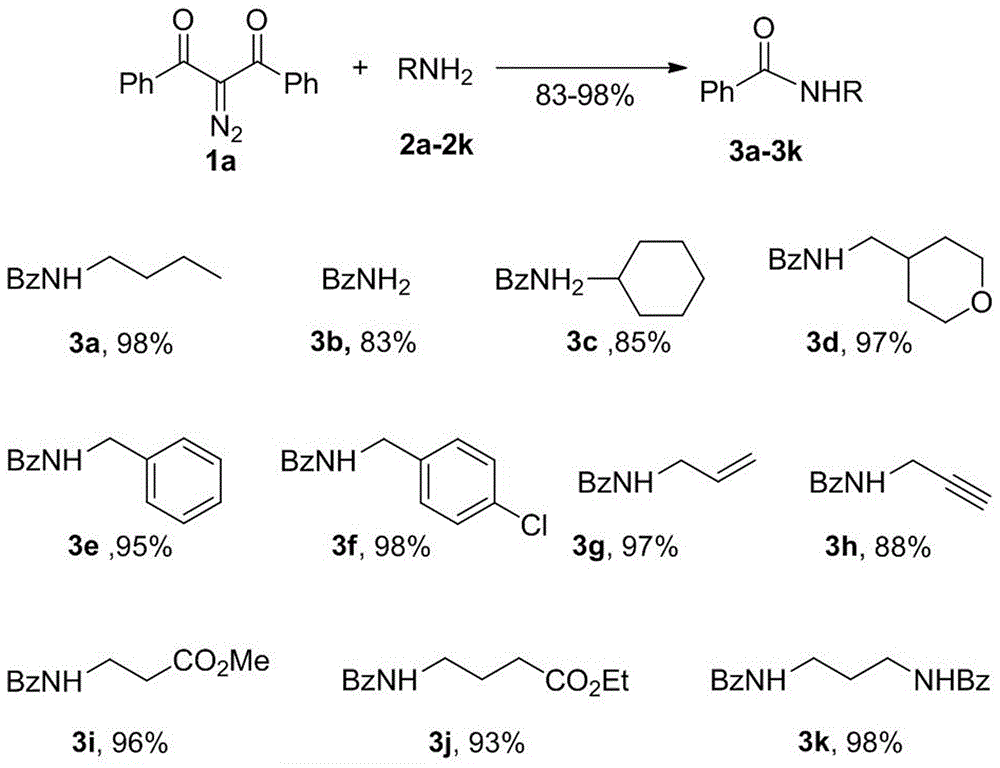
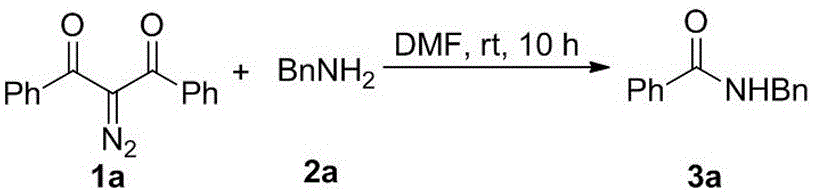Method for preparing amide compound from 2-diazo-1, 3-dicarbonyl compound as acylating agent
A technology for dicarbonyl compounds and amide compounds, applied in the field of preparing amide compounds, can solve the problems of harsh reaction conditions, racemization of chiral compounds, influence and the like, and achieve the effects of mild reaction conditions, simple reaction operation and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0055] The preparation method of N-butylbenzamide is as follows:
[0056] The structural formula is:
[0057]
[0058] Preparation process: Add 2-diazo-1,3-diphenylpropane-1,3-dione (375mg, 1.5mmol) and DMSO (1mL) sequentially into a reaction flask equipped with a stirring bar, and then add to the system Butylamine (1mmol) was added to the mixture, reacted at 20-50°C for 2.0 hours, the reaction was completed, water was added to the system to quench the reaction, and extracted three times with dichloromethane, the organic phases were combined, dried, the solvent was removed, and column chromatography was carried out. Separated to obtain N-butylbenzamide (90%, 160mg) with different substituents on the benzene ring with a purity of 99%
Embodiment 2
[0060] The preparation method of N-(cyclohexylmethyl) benzamide is as follows:
[0061] The structural formula is:
[0062]
[0063] Preparation process: Add 2-diazo-1,3-diphenylpropane-1,3-dione (375mg, 1.5mmol) and 1,4-dioxane ( 1mL), then add cyclohexylamine (1mmol, 113mg) to the system, and react at 20-50°C for 3.0 hours. After the reaction is completed, add water to the system to quench the reaction, and extract three times with dichloromethane, and combine the organic phases , dried, removed the solvent, and subjected to column chromatography to obtain N-(cyclohexylmethyl)benzamide (87%, 189 mg) with a purity of 99%.
Embodiment 3
[0065] The preparation method of N-benzylbenzamide is as follows:
[0066] The structural formula is:
[0067]
[0068] In the formula, R is H, OH, NH 2 , Cl
[0069] Preparation process: Add 2-diazo-1,3-diphenylpropane-1,3-dione (375mg, 1.5mmol)) and DMF (1mL) successively in a reaction flask equipped with a stirring bar, and then add Add substituted benzylamine (1mmol) to the system, and react at 20-50°C for 2.0 hours. After the reaction is completed, add water to the system to quench the reaction, and extract three times with dichloromethane, combine the organic phases, dry, remove the solvent, and Column chromatographic separation was carried out to obtain N-benzylbenzamide compounds with different substituents on the benzene ring with a purity of 99%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com