TRAIL receptor agonists for treatment of fibrotic diseases
A fibrosis and agonist technology, which can be used in skin diseases, bone diseases, respiratory diseases, etc., and can solve problems such as targeting multiple molecules at the same time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0244] Example 1: Expression of TRAIL-R1 / DR4 and TRAIL-R2 / DR5 by Activated Primary Human Hepatic Stellate Cells (HSC) and Pancreatic Stellate Cells (PSC)
[0245] Materials and methods
[0246] Human primary hepatic stellate cells
[0247] Human primary HSC, PSC and stellate cell medium (SteCM) were obtained from ScienCell Research Laboratories (Carlsbad, CA). On polylysine-coated plates, culture in SteCM medium supplemented with 2% FBS, 1% stellate cell growth supplement and 1% penicillin / streptomycin solution cell. Then, in order to activate primary stellate cells, the cells were cultured in 6-well plastic culture plates for 1, 4, 7 and 14 days, and the cells were harvested. Expression of DR-4, DR-5 and α-SMA in cultured astrocytes was determined by Western blot analysis and real-time PCR.
[0248] Comparative quantitative real-time RT-PCR
[0249] Total RNA of cultured cells was extracted with TRIzol reagent (Life Technologies, Grand Island, NY), and reverse transcri...
Embodiment 2
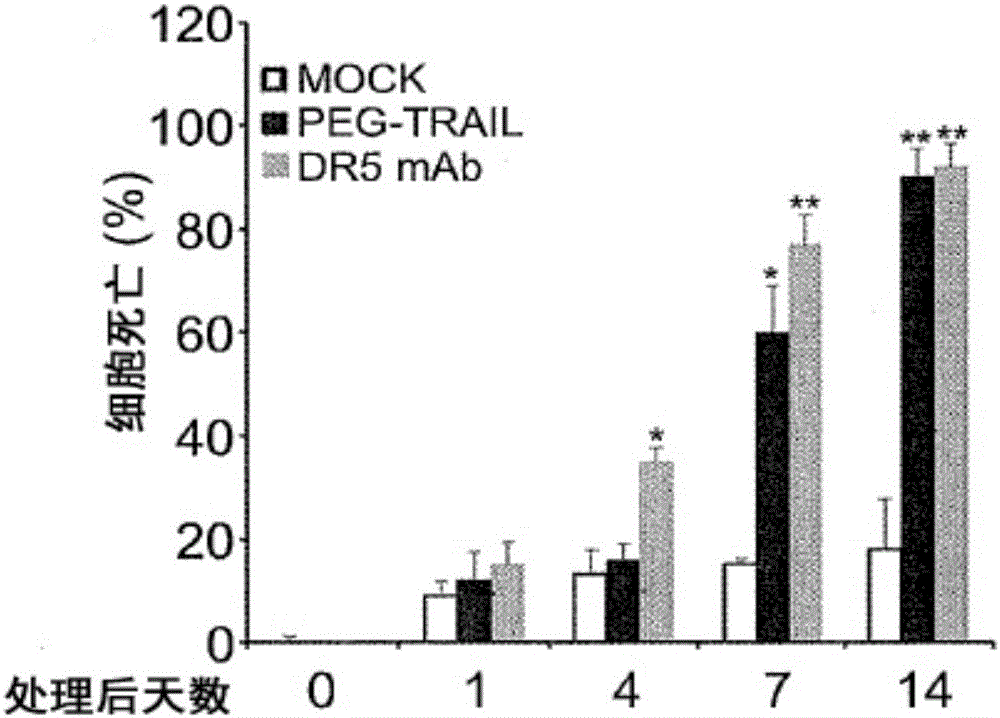
[0254] Example 2: Activated human primary HSCs and PSCs show enhanced sensitivity to TRAIL agonist-induced apoptosis.
[0255] Materials and methods
[0256] Immunofluorescent staining of apoptosis
[0257] Cells were plated on glass coverslips in 35mm dishes (MatTek Corporation, Ashland, MA) and grown for 1, 4, 7 and 14 days. They were then treated with or without TRAIL agonist containing 1 microgram / ml ([mu]g / ml) TRAIL, PEGTRAIL, or DR5 antibody (R&D systems, Minneapolis, MN) at 50 ng / ml for 3 hours on these days. Cells were washed twice with cold PBS and fixed in 4% paraformaldehyde in PBS for 10 min. For detection of apoptosis, TdTIn Situ Apoptosis Detection Kit (TUNEL)-Fluorescein (R&D systems, Gaithersburg, MD) was used according to the manufacturer's instructions. Briefly, cells were incubated with proteinase K for 15 min at room temperature, then washed twice with DW, submerged with TdT labeling buffer, and then incubated with TdT labeling reaction mixture for 1 h...
Embodiment 3
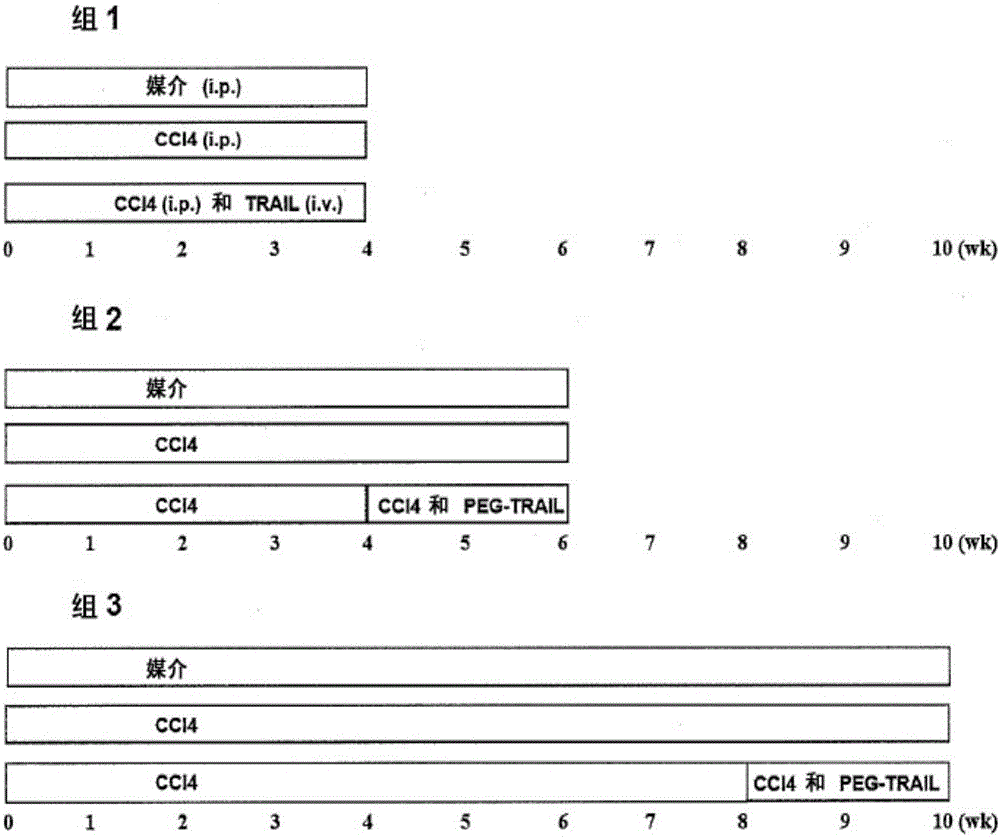
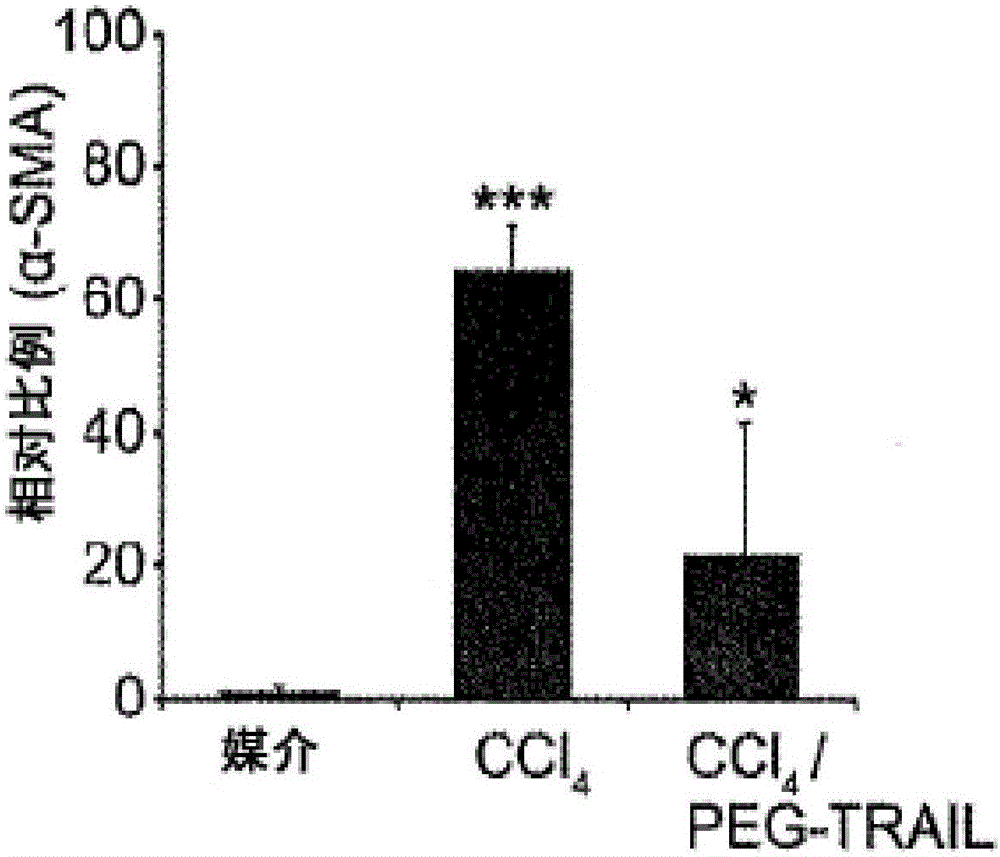
[0265] Example 3: TRAIL treatment prevents liver fibrosis.
[0266] Materials and methods
[0267] in rats via CC1 4 Induced liver fibrosis (group 1)
[0268] SD rats aged 6-8 weeks (Hanlim Experimental Animal Laboratory, Seoul, Korea) were divided into 3 groups (8-10 rats per group); i) vehicle (olive oil), ii) 20 %CCl 4 and iii) CCl in olive oil 4 and TRAIL (group 1, figure 2 ). CCl was administered to rats by intraperitoneal injection three times a week 4 (20% CCl in olive oil 4 , 2ml / kg) or administration of olive oil as a control for 4 weeks, simultaneously treated with TRAIL administered intravenously at 4 mg / kg every 3 days or for the control group (Group 1, figure 2 ) were treated with the same amount of brine.
[0269] Liver histology and immunohistochemistry in liver fibrosis
[0270] After treatment, the animals were sacrificed, and the collected liver tissues were fixed in 10% buffered formaldehyde, embedded in paraffin, and cut into 4 μm thick sectio...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com