Application of triterpene compound and Parkinson's disease treatment medicine
A technology for triterpenoids and compounds, which is applied in the field of Parkinson's drugs and their preparation, can solve the problems of unclear targets and poor efficacy of autophagy inducers, and achieves overcoming the defects of unclear targets and achieving good efficacy. , the effect of a clear target
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
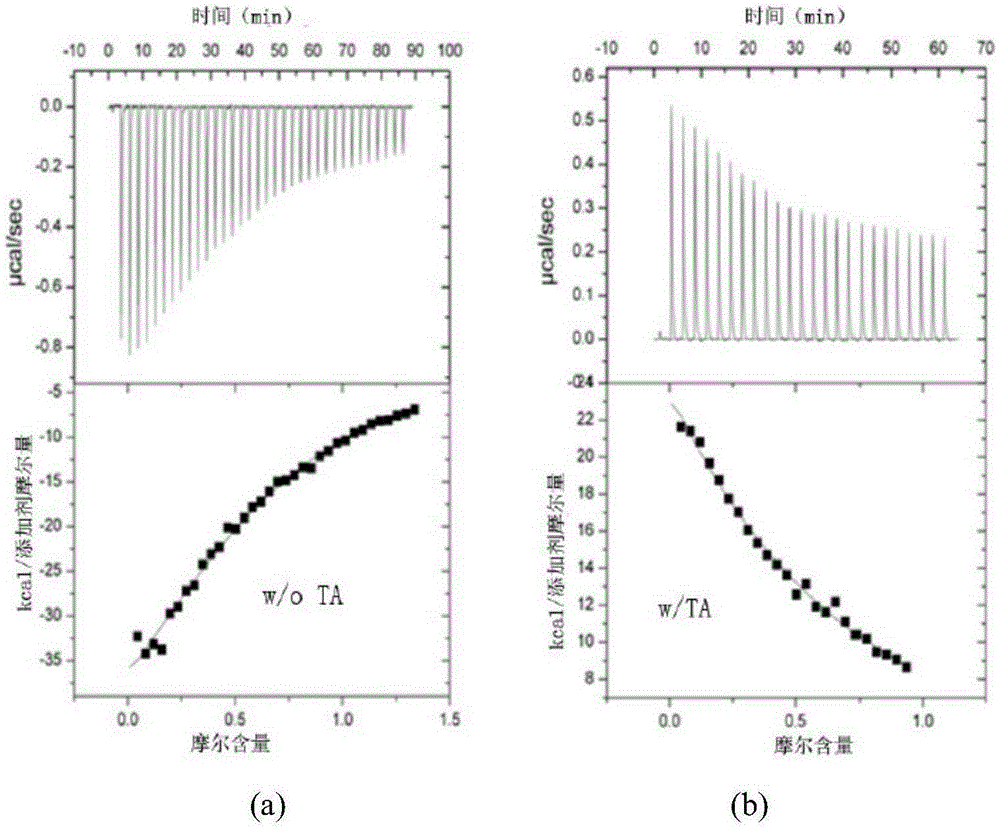
[0049] Effect of isothermal calorimetry (ITC) maslinic acid (MA) and palinginic acid (TA) on the function of autophagy-related proteins:
[0050] The thermal transformation caused by the interaction between the autophagy scaffold protein Beclin1 and the autophagy inhibitor Bcl-2 was measured with an isothermal calorimeter iTC200 (MicroCal) with or without the addition of maslinic acid or palindromic acid. Before the assay, both Beclin1 and Bcl-2 were dialyzed into 50 mM Tris, pH 8.0, and 150 mM NaCl buffer with or without maslinic acid or palinic acid at a final concentration of 10 μM. During the measurement, load 40 μl Beclin1 (concentration 12 mg / ml) on the titration syringe of the isothermal calorimetric titrator, and load 220 μl Bcl-2 (500 μg / ml) on the sample pool of the isothermal calorimetric titrator. Each injection was 2 μl, equilibrated for 180 seconds between each injection. Data were collected and analyzed by Origin 7.0.
[0051] Present embodiment 1 test analysi...
Embodiment 2
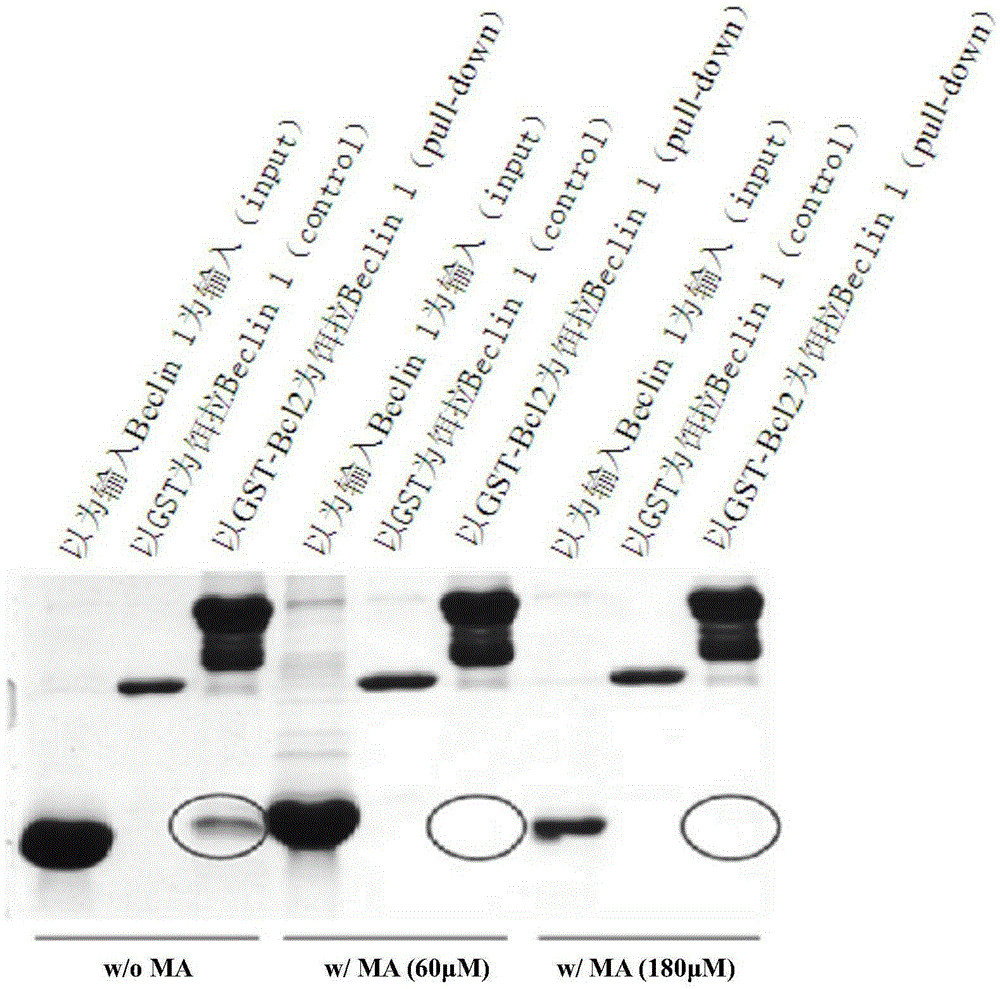
[0054] Pull-down experiments to detect the effects of maslinic acid (MA) and palinginic acid (TA) on the interaction between Beclin1 and autophagy inhibitor Bcl-2:
[0055] GST was used as the fusion tag of the autophagy inhibitor Bcl-2, GST-Bcl-2 was used as bait, and the purified autophagy backbone protein Beclin1 was used as prey to carry out pull-down experiments. Purified GST-Bcl-2 (or GST) 1.0mM and purified Beclin11.0mM (molar concentration 1:1) were mixed in 50mM Tris, pH 8.0, and 150mM NaCl buffer solution, the buffer solution contained or did not contain 10μM final concentration of maslinic acid or palinic acid. The mixture was incubated at room temperature for 30 minutes. The mixture was added to the GST affinity agarose beads equilibrated with the above buffer, and processed by the standard pull-down step. After the protein complex was eluted, the SDS sample buffer was added and separated and detected by SDS-PAGE.
[0056] The result is as Figure 3a , 3b It can...
Embodiment 3
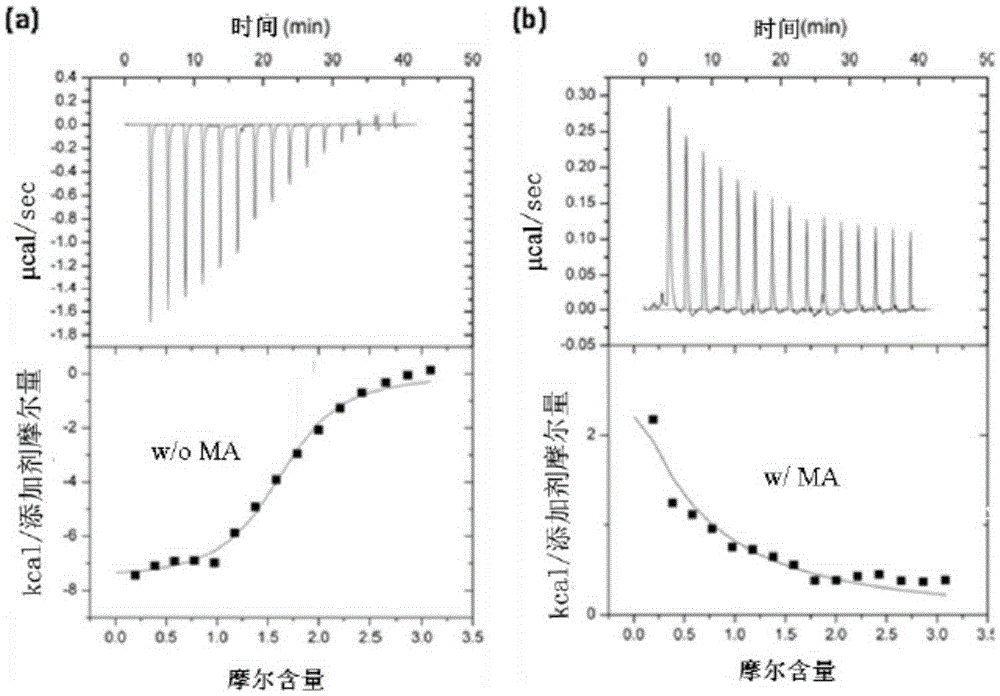
[0058] Light scattering was used to determine the effect of maslinic acid (MA) and palinginic acid (TA) on the interaction between Beclin1 and autophagy inhibitor Bcl-2:
[0059] The protein molecular weight was determined by fast protein liquid chromatography-static light scattering; the protein particle size was detected by dynamic light scattering. Purified GST-Bcl-2 (or GST) 1.0mM and purified Beclin11.0mM (molar concentration 1:1) mixed in 50mM Tris, pH 8.0, and 150mM NaCl buffer, the buffer contains or does not contain a final concentration of 10μM Maslinic acid or Potentinic acid. The mixture was incubated at room temperature for 30 minutes. The mixture is loaded onto a gel chromatography column, separated and purified by fast protein liquid chromatography, and the molecular weights of components with different peak times are detected by a static light scattering instrument; or the particle size of the protein is detected by a dynamic light scattering instrument. The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
| Particle size | aaaaa | aaaaa |
| Protein molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com