Method for synthesizing rutile phase titania photocatalyst containing electron-trapped oxygen vacancies
A technology of rutile phase and titanium dioxide, which is applied in the direction of physical/chemical process catalysts, chemical instruments and methods, chemical/physical processes, etc., can solve the problems of constraints, complicated preparation process, high equipment cost and production cost, and achieve uniform size and high production cost. Simple process and good reproducibility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0026] Dissolve 0.2 g of polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer P123 and 2.3 g of titanium tetraisopropoxide TTIP in 0.7 mL of concentrated hydrochloric acid, 2.35 mL of isopropanol, and 0.15 mL of deionized After the mixed solution was evaporated in an evaporating dish at room temperature for 12 hours, it was transferred to a hydrothermal reaction kettle and crystallized at 180°C for 12 hours. After the reaction was completed, the crystallized product was suction-filtered and dried naturally to obtain the target product.
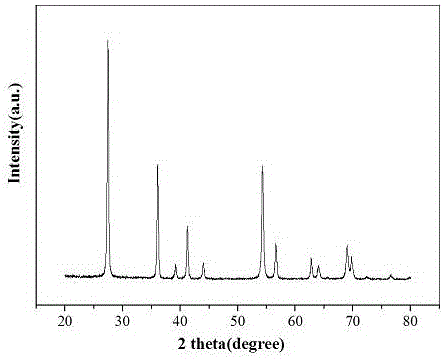
[0027] According to attached figure 1 In the X-ray (XRD) spectrum of titanium dioxide photocatalyst, it can be seen that the diffraction peaks belong to the crystal structure of rutile phase titanium dioxide (JCPDS card number: 21-1276).
Embodiment 2
[0029] Dissolve 0.2 g of polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer P123 and 2.3 g of titanium tetraisopropoxide TTIP in 0.7 mL of concentrated hydrochloric acid, 2.35 mL of isopropanol, and 0.15 mL of deionized Then the mixed solution was evaporated in an evaporating dish at room temperature for 24 hours, then transferred to a hydrothermal reaction kettle for crystallization at 180°C for 24 hours, after the reaction was completed, the crystallized product was suction filtered and dried naturally to obtain the target product.
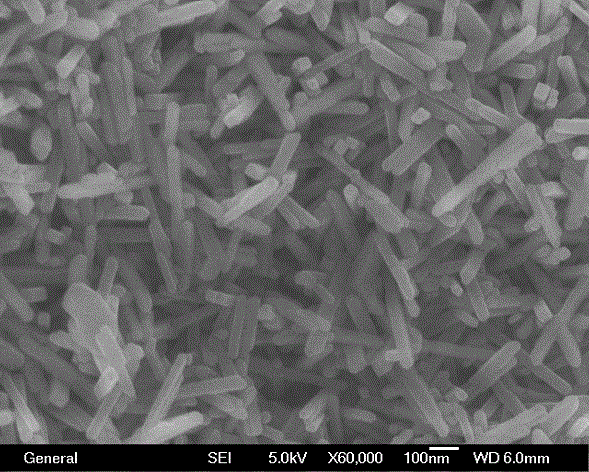
[0030] According to attached figure 2 The scanning electron microscope (SEM) of the titanium dioxide photocatalyst shows that the morphology of the prepared sample is very regular nanorods, about 200-250nm in length.
Embodiment 3
[0032] Dissolve 0.2 g of polyethylene oxide-polypropylene oxide-polyethylene oxide triblock copolymer P123 and 2.3 g of titanium tetraisopropoxide TTIP in 0.7 mL of concentrated hydrochloric acid, 2.35 mL of isopropanol, and 0.15 mL of deionized After the mixed solution was evaporated in an evaporating dish at room temperature for 24 hours, it was transferred to a hydrothermal reaction kettle and crystallized at 180°C for 48 hours. After the reaction was completed, the crystallized product was suction-filtered and dried naturally to obtain the target product. product.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com