Method for synthesizing disodium octaborate tetrahydrate by utilizing liquid boron ore
A technology of disodium octaborate tetrahydrate and boron ore, which is applied in the direction of borate, boron compound, boron oxide compound, etc., can solve the problems of high impurity content, low boron content, high energy consumption, etc., to simplify the preparation process and product The effect of good quality and reduced energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment 1
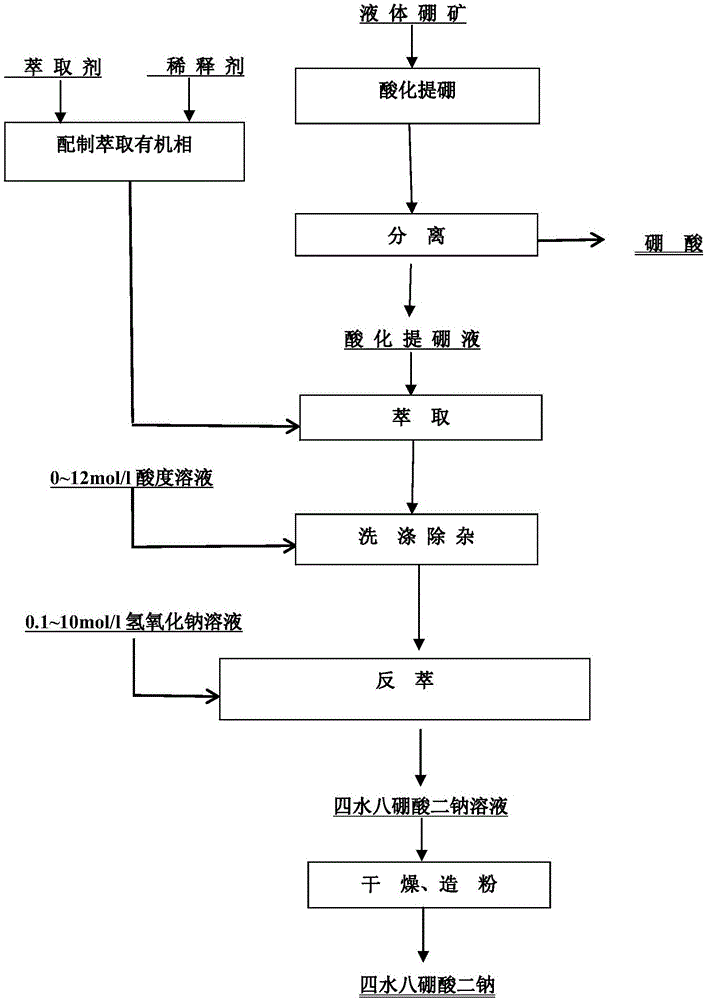
[0033] Isooctyl alcohol is selected as the alcohol extractant, sulfonated kerosene is used as the diluent to form the extracted organic phase, and the volume ratio of isooctanol in the extracted organic phase is 20% to 50%.
[0034]Add 6mol / l hydrochloric acid solution to 1 part of liquid boron ore, react until the pH value of the solution is ≤2, filter the above solution to obtain boric acid solid and acidified boron extraction solution, and the flow ratio of extraction organic phase agent and acidification boron extraction solution is 1: 1~1.5, carry out 4-stage countercurrent extraction at 25°C, obtain boron-rich organic phase after phase separation, use 1mol / l hydrochloric acid as detergent, flow ratio of boron-rich organic phase to detergent is 1:20~30, at 25°C Perform 3-stage countercurrent washing, and obtain the boron-rich organic phase after washing and removing impurities after phase separation. Using 0.5-1mol / l sodium hydroxide solution as the stripping agent, the fl...
specific Embodiment 2
[0036] The alcohol extractant is a compound system of isooctyl alcohol and 2-ethyl-1, 3-hexanediol, and the diluent is sulfonated kerosene to form the extraction organic phase. The volume ratio of isooctyl alcohol in the extraction organic phase is 10% to 50%. %, 2-ethyl-1,3-hexanediol accounts for 10%-50% by volume in the extracted organic phase, and sulfonated kerosene accounts for 50%-70% by volume in the extracted organic phase.
[0037] Add 3 mol / l sulfuric acid solution to 1 part of liquid boron ore, react until the pH value of the solution is ≤2, filter the above solution to obtain boric acid solid and acidified boron extraction solution, and the flow ratio of the extraction organic phase agent to the acidification boron extraction solution is 1: 1~2, carry out 3-stage countercurrent extraction at 25°C, obtain boron-rich organic phase after phase separation, use 3mol / l hydrochloric acid as detergent, flow ratio of boron-rich organic phase to detergent is 1:20~30, and ext...
specific Embodiment 3
[0039] Isooctyl alcohol is selected as the alcohol extractant, sulfonated kerosene is used as the diluent to form the extracted organic phase, and the volume ratio of isooctanol in the extracted organic phase is 20% to 50%.
[0040] Add 6mol / l hydrochloric acid solution to 1 part of liquid boron ore, react until the pH value of the solution is ≤2, filter the above solution to obtain boric acid solid and acidified boron extraction solution, and the flow ratio of extraction organic phase agent and acidification boron extraction solution is 1: 1~1.5, carry out 4-stage countercurrent extraction at 25°C, obtain boron-rich organic phase after phase separation, use 1mol / l hydrochloric acid as detergent, flow ratio of boron-rich organic phase to detergent is 1:20~30, at 25°C Perform 3-stage countercurrent washing, and obtain the boron-rich organic phase after washing and removing impurities after phase separation. Using 1.5-2mol / l sodium hydroxide solution as the stripping agent, the f...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com