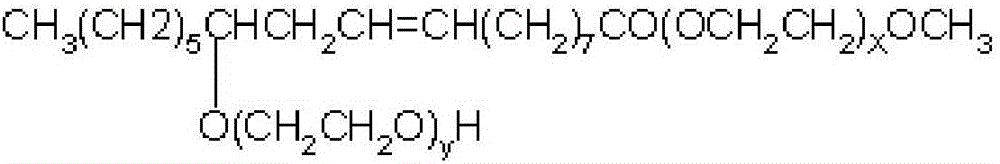Method for synthesizing methyl ricinoleate ethoxylate sulfonate
A technology of ethoxylate and ricinoleic acid, which is applied in the direction of sulfonate preparation, chemical instruments and methods, chemical/physical processes, etc., to achieve the effects of low reaction temperature, high conversion rate and short reaction time
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
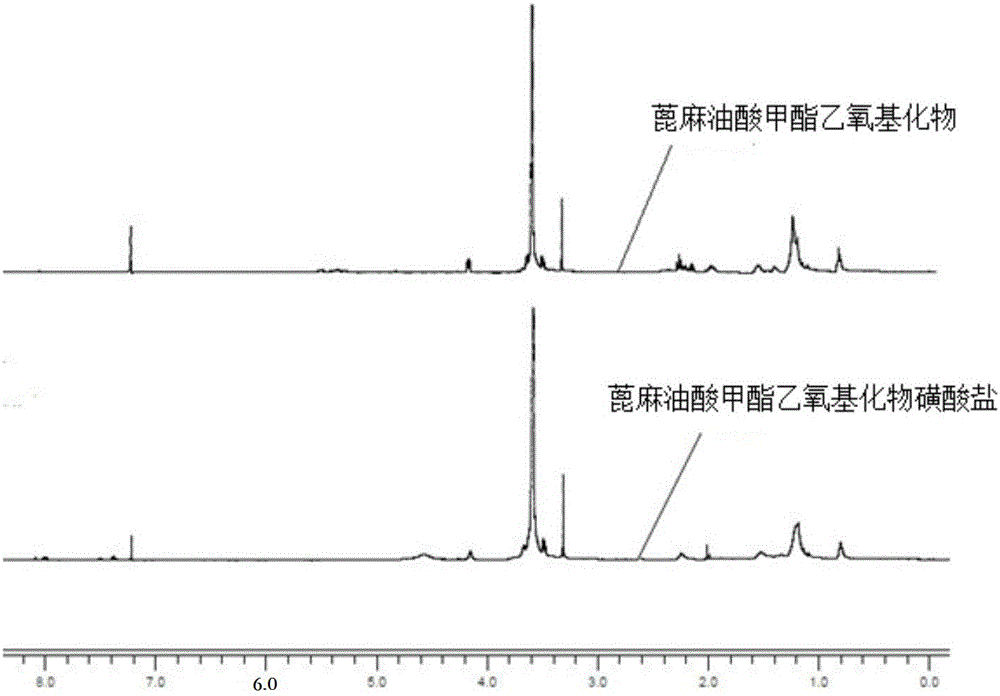
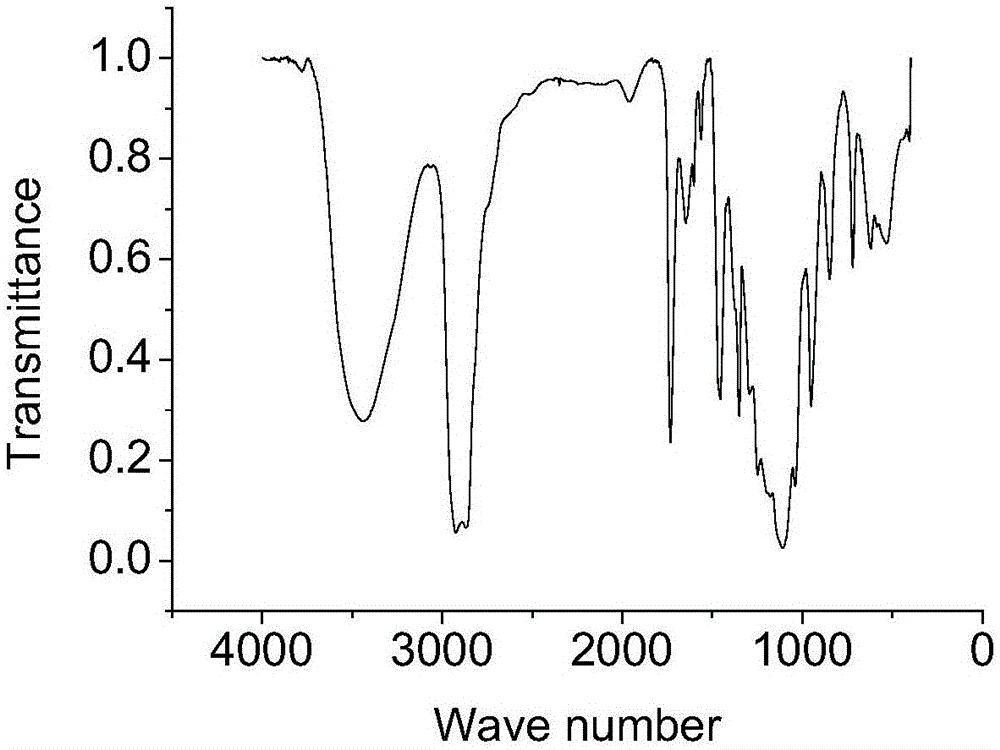
[0028] 30g (0.046mol) ricinoleic acid methyl ester ethoxylate (average molecular weight 1151, X average value is 13.2, Y average value is 5.9, iodine value is 22.5g / 100g), 100g deionized water, 100g isopropanol , 8.7g (0.084mol) sodium bisulfite, 1.5g tert-butyl peroxybenzoate and 0.13g ferric trichloride were added in a 500ml four-necked flask (ferric trichloride and sulfurous acid should be mixed in advance before feeding) Sodium hydrogen was dissolved in deionized water respectively), heated to 40°C, and reacted for 2h. After the reaction, the water and isopropanol were removed by rotary evaporation, dissolved in excess absolute ethanol, the salt was removed by hot filtration, and the ethanol was removed by rotary evaporation to obtain the product. The main indexes of the product are as follows: (1) the iodine value is 11.8g / 100g; (2) the mass fraction of ricinoleic acid methyl ester ethoxylate sodium sulfonate is 48.2%.
Embodiment 2
[0030] With 20g (0.031mol) methyl ricinoleic acid ethoxylate (average molecular weight 1151, X mean value is 13.2, Y mean value is 5.9, iodine value is 22.5g / 100g), 20g deionized water, 20g methanol, 3.6 g (0.035mol) sodium bisulfite, 1.2g tert-butyl peroxybenzoate and 0.11g ferric trichloride are added in a 250ml four-necked flask (ferric trichloride and sodium bisulfite should be mixed in advance before feeding were dissolved in deionized water), heated to 50°C, and reacted for 2.5h. After the reaction, the water and methanol were removed by rotary evaporation, dissolved with excess absolute ethanol, the salt was removed by hot filtration, and the ethanol was removed by rotary evaporation to obtain the product. The main indexes of the product are as follows: (1) the iodine value is 17.7g / 100g; (2) the mass fraction of ricinoleic acid methyl ester ethoxylate sodium sulfonate is 22.1%.
Embodiment 3
[0032] 30g (0.041mol) ricinoleic acid methyl ester ethoxylate (average molecular weight 739, X average value is 7.9, Y average value is 1.8, iodine value is 35.3g / 100g), 90g deionized water, 90g ethanol, 10g (0.096mol) sodium bisulfite, 1.6g tert-butyl peroxybenzoate and 0.15g ferric trichloride are added in the four-necked flask of 500ml (the ferric trichloride and sodium bisulfite should be separated in advance before feeding) dissolved in deionized water), heated to 40°C, and reacted for h. After the reaction, the water and ethanol were removed by rotary evaporation, dissolved with excess absolute ethanol, the salt was removed by hot filtration, and the ethanol was removed by rotary evaporation to obtain the product. The main indexes of the product are as follows: (1) the iodine value is 14.2g / 100g; (2) the mass fraction of ricinoleic acid methyl ester ethoxylate sodium sulfonate is 58.5%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Iodine value | aaaaa | aaaaa |
| Iodine value | aaaaa | aaaaa |
| Iodine value | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com