Pimavanserin preparation method
A technology of pimaserin and solvent, which is applied in the fields of chemistry and pharmacy, and can solve problems such as limited industrialization, less reports of hallucinations of touch and taste, and auditory hallucinations
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
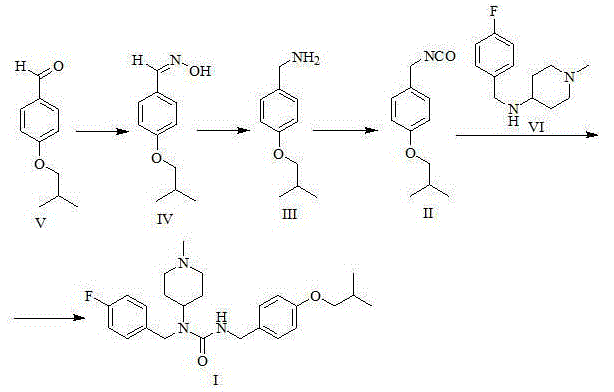
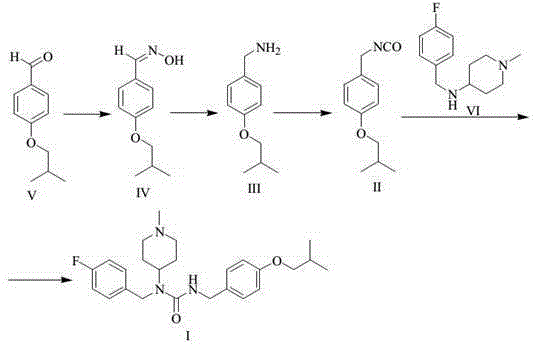
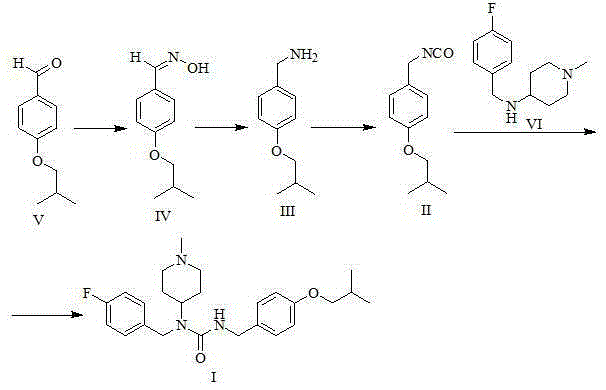
[0035] Preparation of 4-isobutoxybenzaldehyde oxime (formula IV)
[0036]
[0037] Take a 20L reaction bottle, add 1.00kg (5.61mol) 4-isobutoxybenzaldehyde (Formula V) and 10L ethanol respectively, add 1.5L (11.25mol) 30% sodium hydroxide aqueous solution under stirring, and finally add 0.47 kg (6.76mol) of hydroxylamine hydrochloride, after the addition, stir and react at room temperature for one hour at 25°C. Concentrate under reduced pressure at 50°C to remove ethanol. A large amount of solids precipitate out of the concentrate. Add 10L of water, stir well for 1 hour, filter with suction, and wash the filter cake with 2L of water. The solid was dried under reduced pressure at 70°C for 24 hours to obtain 1.05 kg of the product (yield 96.8%).
Embodiment 2
[0039] Preparation of 4-isobutoxybenzylamine (formula III)
[0040]
[0041] method one
[0042]Take a 20L three-necked bottle, dissolve 1.0kg of 4-isobutoxybenzaldehyde oxime (Formula IV) in 10L of methanol, and then add 100g of 10% palladium carbon. Put it into a 40°C water bath and heat it, add 3.0kg of ammonium formate while stirring, and keep it warm at 40°C for 2 hours. After filtration, the filtrate was concentrated to dryness under reduced pressure at 40°C. Add 10L of water and 5L of methyl tert-butyl ether to the concentrate, separate the layers, and extract the aqueous phase with 5L of methyl tert-butyl ether. The organic phases were combined, washed with 50ml of saturated brine, dried with 0.5kg of anhydrous sodium sulfate, filtered, and the filtrate was concentrated to dryness at 40°C under reduced pressure to obtain 0.81kg of 4-isobutoxybenzylamine (yield 87.3%).
[0043] Method Two
[0044] Take a 250mL three-necked bottle, dissolve 10.0g of 4-isobutox...
Embodiment 3
[0050] Preparation of pimaserin (formula I)
[0051] a) Preparation of 4-isobutoxybenzyl isocyanate (formula II)
[0052]
[0053] Under nitrogen protection, 10 L of toluene and 828 g (2.79 mol) of trimeric phosgene were sequentially added to a 30 L reaction flask equipped with mechanical stirring. After stirring until the solid dissolved, the temperature of the reaction solution was lowered to below 0°C. Dissolve 1 kg (5.58 mol) of 4-isobutoxybenzylamine (Formula III) and 867 g (8.37 mol) of triethylamine in 5 L of toluene, and add dropwise to the above reaction solution under temperature control -10°C-0°C , The dropwise addition time is about 3-4 hours. After the dropwise addition is completed, the temperature is raised to 25° C. for 1 hour, and then heated to reflux for 5 hours. The reaction solution was lowered to room temperature, and 10 L of water was added to the reaction solution, and the layers were washed, and the organic phase was washed with 10 L of saturated ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com