10-HCPT (10-hydroxycamptothecine) derivative, synthesis method and application thereof
A technology of hydroxycamptothecin and its synthesis method, which is applied in the field of camptothecin drugs, can solve the problems of slow crossing biomembrane speed, limitation of clinical application, and large tissues and organs, so as to increase the speed and quantity, and improve the utilization rate of drugs , reduce the effect of toxic and side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] In a second aspect, the present invention provides a method for synthesizing 10-hydroxycamptothecin derivatives, comprising the following steps: dissolving 10-hydroxycamptothecin and a carboxylic acid with the structure RCOOH in pyridine, wherein R is any Select a substituted C1-C10 straight chain alkyl or branched chain alkyl, and / or, an optionally substituted C2-C10 alkenyl; then add EDCI and stir for 1-4 hours to obtain the 10-hydroxycamptothecin base derivatives.
[0060] Among them, EDCI is carbodiimide.
[0061] In a preferred embodiment, the synthesis method further includes: after stirring for 1-4 hours, adding dichloromethane and water, stirring for 10-50 minutes, separating the organic layer, and finally drying and concentrating the residue Purify and elute with dichloromethane and methanol at a volume ratio of (5-25):1 to obtain the 10-hydroxycamptothecin derivative.
[0062] In a preferred embodiment, the following steps are included: dissolving 1-4 mmol o...
Embodiment 1
[0068] A 10-hydroxycamptothecin derivative 1, the structural formula is as follows:
[0069]
Embodiment 2
[0071] The derivative described in embodiment 1 is synthesized by the following method:
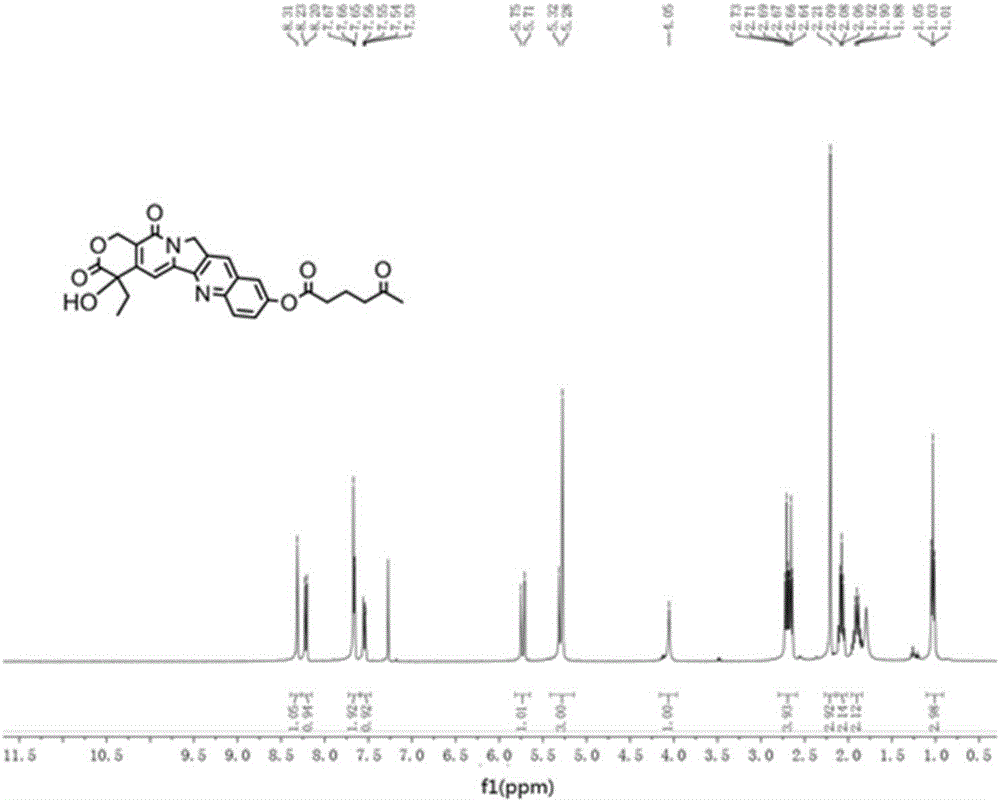
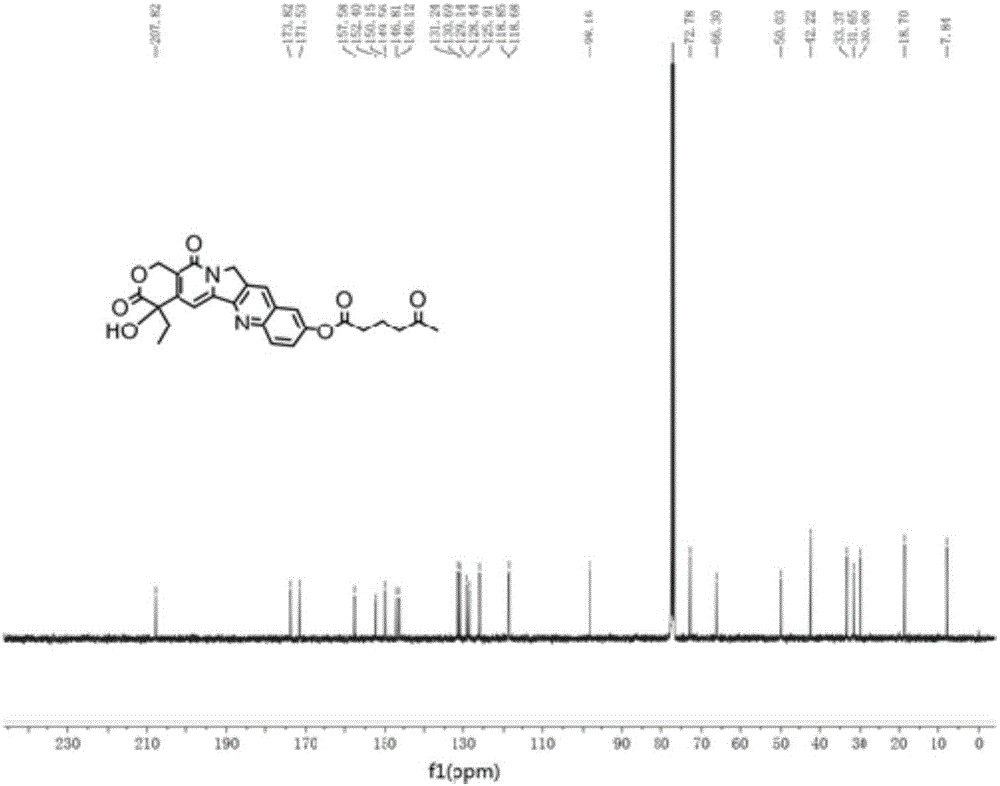
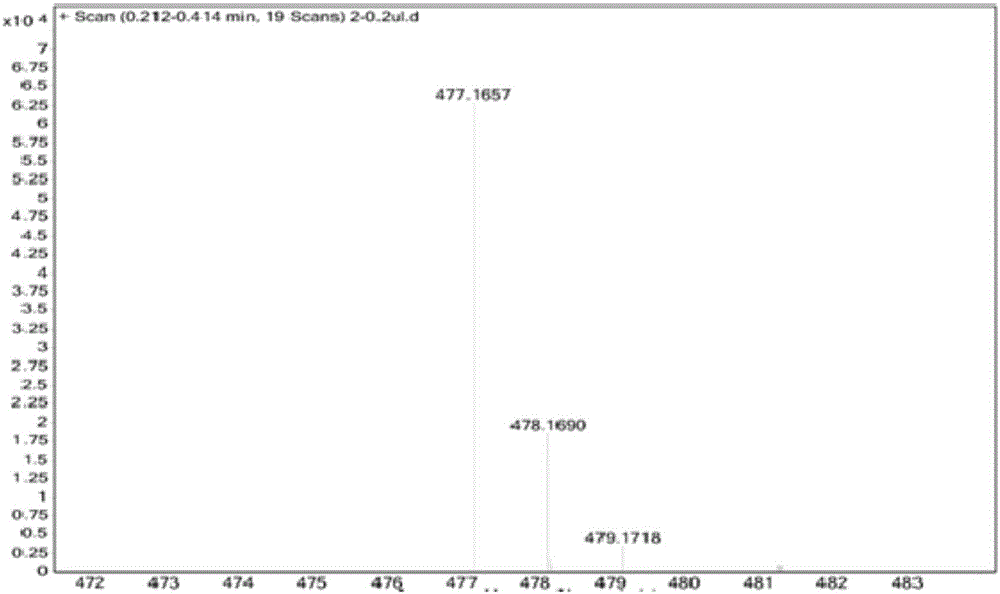
[0072] 10-HCPT (1.00g, 2.7mmol) and 5-carbonylhexanoic acid (1.07g, 8.1mmol) were dissolved in 10mL of pyridine, EDCI (2.06g, 10.8mmol) was added at room temperature, stirred for 3h, thin-layer chromatography Monitor the response. Add 50mL of dichloromethane and 50mL of water to the reaction system, stir and react for 30 minutes, separate the organic layer, dry and concentrate under reduced pressure, and purify the obtained residue with a silica gel column, elute with dichloromethane and methanol, dichloromethane and The volume ratio of methanol was 10:1, and a yellow solid compound, namely 10-hydroxycamptothecin derivative 1 (1.25 g, yield 96%) was obtained.
[0073] The synthesis reaction is as follows:
[0074]
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com