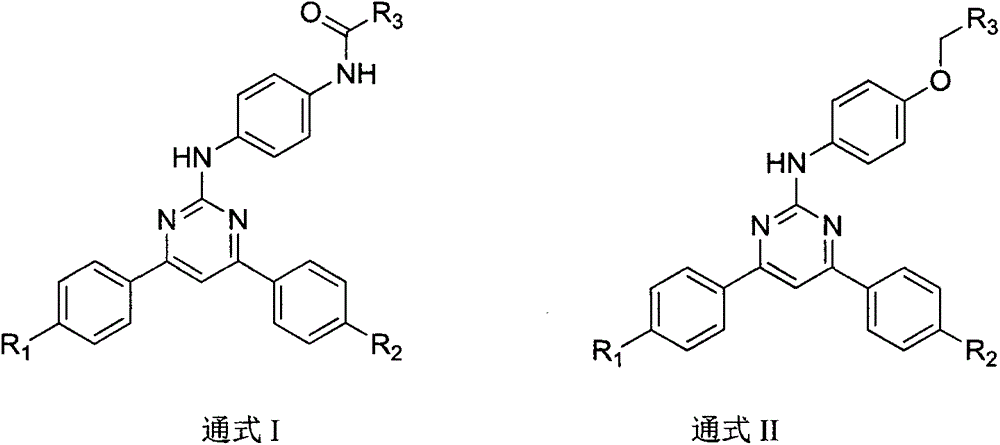
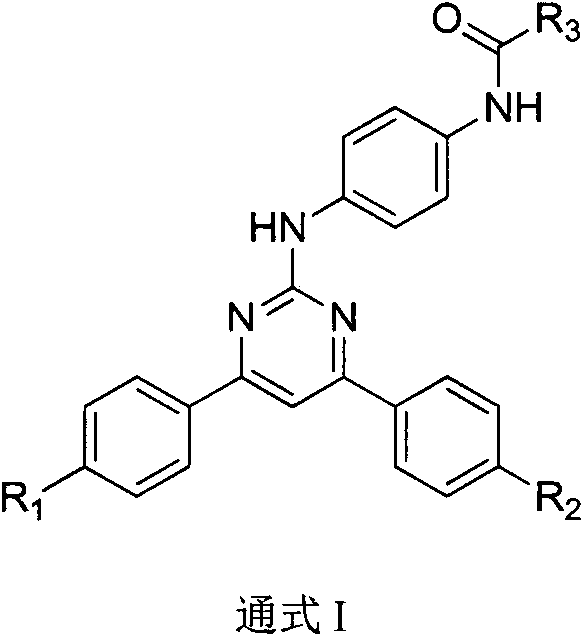
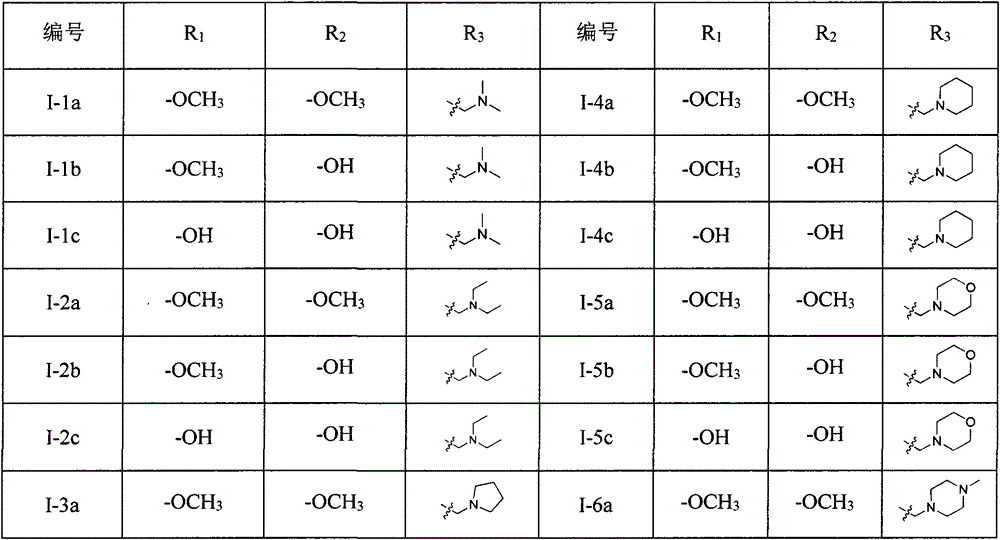
4,6-dibenzyl pyrimidine compounds, preparing method thereof and medical uses of the compounds
A compound and pharmaceutical technology, applied in the field of medicinal chemistry, can solve the problems that the clinical treatment effect is not as good as Tamoxifen, endometrial cancer and drug resistance, venous thrombosis, vasodilation, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
specific Embodiment approach
[0054] The specific embodiment (described embodiment is only used to illustrate the present invention, is not used to limit the present invention)
[0055] The preparation of some compounds is carried out as follows:
[0056] 1 H-NMR nuclear magnetic resonance was determined by Bruker AV300 (300MHz) nuclear magnetic resonance instrument (TMS was the internal standard), and mass spectrometry was determined by Shimadzu GC / MS-QP2010 mass spectrometer (EI-MS), Agilent1100LC-MSD-Trap / SL type mass spectrometer (ESI-MS) measurement.
[0057] The silica gel used for column chromatography is 100-200 mesh, 200-300 mesh or 300-400 mesh silica gel (Qingdao Ocean Chemical Factory), and the eluent is petroleum ether-ethyl acetate system or chloroform-methanol system. Thin-layer chromatography (TLC) uses a GF254 thin-layer chromatography plate (Yantai Jiangyou Silica Gel Development Co., Ltd.); the TLC development system is a petroleum ether-ethyl acetate system or a chloroform-methanol sy...
Embodiment 1
[0059] Synthesis of 4,6-bis(4-methoxyphenyl)-3,4-dihydropyrimidin-2(1H)one (4)
[0060] Dissolve 4-methoxybenzaldehyde (0.4mL, 3.33mmol) in 12.5mL of acetonitrile, add hexahydrate, ferric chloride (90mg, 0.333mmol), 4-methoxyacetophenone (0.5 g, 3.33mmol), urea (0.3g, 5mmol) and trimethylchlorosilane (0.417mL, 3.33mmol), heated to 80°C under nitrogen protection and refluxed for 10h. After cooling to room temperature, the reaction solution was stirred with 50 mL of water, filtered, and the filter cake was washed with diethyl ether to obtain a light yellow solid 4 (0.6 g, 58%). MS (ESI, m / z): 311 [M+H] + .
Embodiment 2
[0062] Synthesis of 2-chloro-4,6-bis(4-methoxyphenyl)pyrimidine (5)
[0063] Compound 4 (1 g, 3.2 mmol) and 4 mL of phosphorus oxychloride were added to a 25 mL flask, and the temperature was raised to 110° C. for 5 h under reflux. Stop heating, cool to room temperature, and slowly add 50 mL of crushed ice. Extracted with ethyl acetate, the organic layer was washed with saturated brine, dried over anhydrous sodium sulfate, concentrated to dryness under reduced pressure, column chromatography gave light yellow solid 5 (0.6 g, 58%). MS (ESI, m / z): 327 [M+H] + .
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com