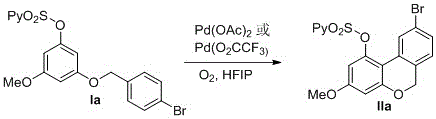
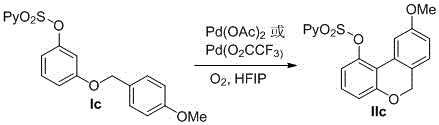Synthetic method for cannabinol compound
A synthetic method and compound technology, applied in the field of synthesis of cannabinol compounds, can solve the problems of high reagent price, lengthy steps, and low efficiency, and achieve the effects of high atom utilization, simple operation, and high reaction efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0022] The synthetic route of embodiment 1 cannabinol is as follows:
[0023]
[0024] Compound 2 Synthesis
[0025] In a 50mL single-necked flask, 3,5-dihydroxypentylbenzene (1.80g, 10 mmol), 4-methylbenzyl bromide (1.85g, 10 mmol), K 2 CO 3 (1.38g, 10 mmol), acetone 30ml, and the reaction mixture was stirred evenly at room temperature. React at 50° C. for 14 h, and detect the reaction with a TLC plate until the reaction of the raw materials is complete. Concentration under reduced pressure, separation by column chromatography, petroleum ether / ethyl acetate as eluent at 10:1, to obtain product 2 (2.28g, 80%)
[0026] IR (KBr): 3032, 2917, 2411, 1462, 1387, 1127, 1055, 932, 776 cm -1 ; 1 H NMR (400 MHz, CDCl 3 ) δ 7.32 – 7.30 (m, 2H), 7.20 – 7.18 (m, 2H), 6.41 (s, 1H),6.30 – 6.27 (m, 2H), 4.96 (s, 2H), 4.84 (s, 1H), 2.68 – 2.44 (m, 2H), 2.36 (s, 3H), 1.72 – 1.48 (m, 2H), 1.29 – 1.25 (m, 4H), 0.88 (t, J = 6.6 Hz, 3H). 13 C NMR (100 MHz, CDCl 3 ) 13 C NMR (100...
Embodiment 2
[0044] Add palladium trifluoroacetate or palladium acetate (10 mmol%) into a 10 mL single-necked flask, seal the rubber stopper and evacuate, replace the air in the bottle with an oxygen balloon, and fill the bottle with oxygen. Then, hexafluoroisopropanol (10.0 mL) was sequentially added to the flask via a syringe under an oxygen atmosphere, and the reaction mixture was stirred homogeneously at room temperature. Add Ia (1mmol) to the well-stirred flask, react at room temperature for 24h (reaction time and temperature are determined by different substrates), TLC plate detection reaction, until the reaction of raw materials is complete. The coupling product IIa was separated by column chromatography (yield 75%) as a white solid; melting point: 151-152°C. IIa
[0045]
[0046] IR (KBr):2922, 1728, 1620, 1464, 1379, 1196, 1049, 773, 592cm -1 ; 1 H NMR (400 MHz, CDCl 3 ) δ 8.54 – 8.53 (m, 1H), 8.01 (d, J = 7.9 Hz, 1H),7.93 (d, J =1.8 Hz, 1H),7.82 (td, J = 7.8, 1.7 Hz...
Embodiment 3
[0049] Add palladium trifluoroacetate or palladium acetate (10 mmol%) into a 10 mL single-necked flask, seal the rubber stopper and evacuate, replace the air in the bottle with an oxygen balloon, and fill the bottle with oxygen. Then, hexafluoroisopropanol (10.0 mL) was sequentially added to the flask via a syringe under an oxygen atmosphere, and the reaction mixture was stirred homogeneously at room temperature. Add Ib (1mmol) to the well-stirred flask, react at room temperature for 24h (the reaction time and temperature are determined by different substrates), and check the reaction with a TLC plate until the raw materials are completely reacted. The coupling product IIb was separated by column chromatography (yield 80%). White solid; melting point: 205-206 ℃.
[0050]
[0051] IR (KBr): 3435, 2960, 1687, 1603, 1379, 1257, 1174, 1111, 1022, 806,590 cm -1 1 H NMR (400 MHz, CDCl 3 ) δ 8.43 – 8.28 (m, 1H), 8.01 (d, J = 7.2 Hz,1H), 7.72 (d, J = 7.9 Hz, 1H), 7.66 – 7.58...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com