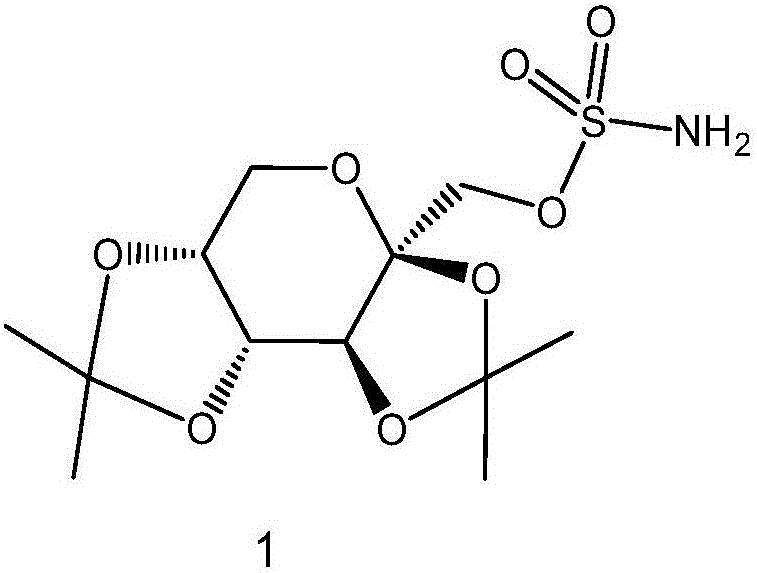
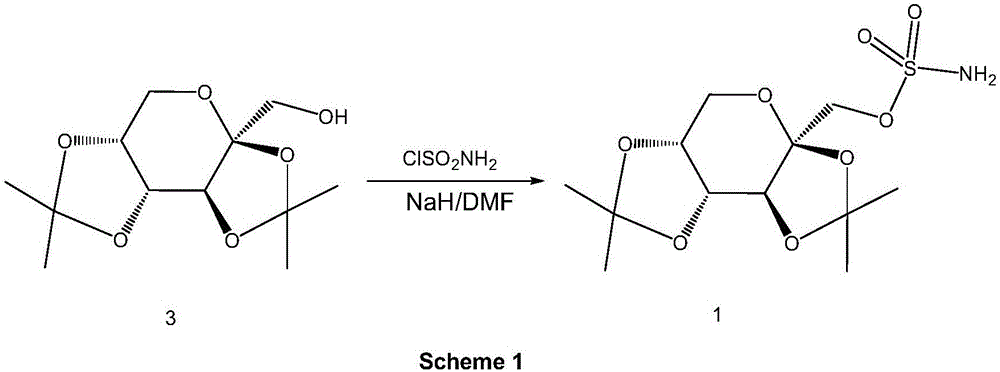
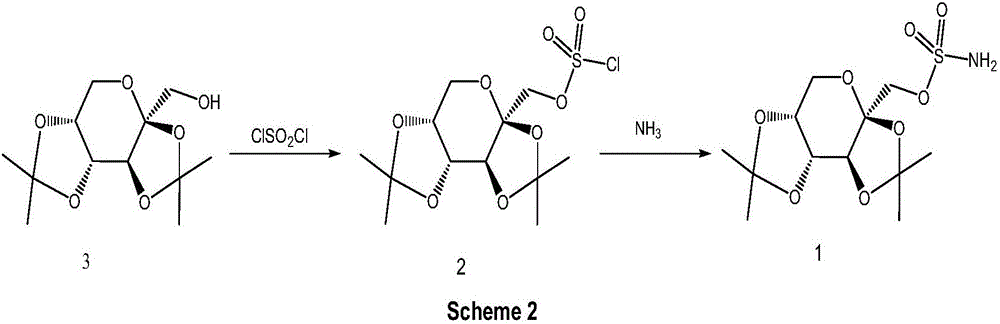
Preparation method of high-purity topiramate
A topiramate, high-purity technology, applied in the field of preparation of high-purity topiramate, can solve the problems of poor controllability, limited sources, potential safety hazards and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Add 17.1 g of sulfuryl chloride and 140 ml of toluene to a 500 ml three-necked flask at room temperature and cool down to -10°C. Add dropwise a mixed solution of 30 g of fructose diacetone, 140 ml of toluene, and 11 g of pyridine. The dropwise addition temperature does not exceed 5° C., and the ice bath is removed after the dropwise addition. The temperature was naturally raised and stirred for 2 hours, and 140 ml of water was added to wash the organic phase. The organic layer was washed successively with 90 ml of 10% hydrochloric acid, 90 ml of purified water, 90 ml of saturated sodium bicarbonate, and 90 ml of saturated sodium chloride. Toluene was distilled off under reduced pressure to obtain 42 g of light yellow oil.
[0033] Dissolve the light yellow oil obtained in the previous step in 300ml tetrahydrofuran, pass ammonia gas at 20-30°C for 5-7 hours, filter off the solid formed by the reaction, rinse the filter cake with 30ml tetrahydrofuran, combine the organic...
Embodiment 2
[0036] Add 17.1 g of sulfuryl chloride and 140 ml of toluene to a 500 ml three-necked flask at room temperature and cool down to -10°C. Add dropwise a mixed solution of 30 g of fructose diacetone, 140 ml of toluene, and 11 g of pyridine. The dropwise addition temperature does not exceed 5° C., and the ice bath is removed after the dropwise addition. The temperature was naturally raised and stirred for 2 hours, and 140 ml of water was added to wash the organic phase. The organic layer was successively washed with 90 ml of 10% hydrochloric acid, 90 ml of purified water, 90 ml of saturated sodium bicarbonate, and 90 ml of saturated sodium chloride. Toluene was distilled off under reduced pressure to obtain 42 g of light yellow oil.
[0037] Dissolve the light yellow oil obtained in the previous step in 300ml tetrahydrofuran, pass ammonia gas at 20-30°C for 5-7 hours, filter off the solid generated by the reaction, rinse the filter cake with 30ml tetrahydrofuran, combine the orga...
Embodiment 3
[0040] Add 17.1 g of sulfuryl chloride and 140 ml of dichloroethane to a 500 ml three-necked flask at room temperature and cool down to -10°C. Add dropwise a mixed solution of 30 g of fructose diacetone, 140 ml of dichloroethane, and 11 g of pyridine. The dropwise addition temperature does not exceed 5° C., and the ice bath is removed after the dropwise addition. The temperature was naturally raised and stirred for 2 hours, and 140 ml of water was added to wash the organic phase. The organic layer was successively washed with 90 ml of 10% hydrochloric acid, 90 ml of purified water, 90 ml of saturated sodium bicarbonate, and 90 ml of purified water. Dichloroethane was distilled off under reduced pressure to obtain 41.5 g of light yellow oil.
[0041] Dissolve the light yellow oil obtained in the previous step in 300ml of dichloroethane, pass ammonia gas at 20-30°C for 7 hours, filter off the solid generated by the reaction, rinse the filter cake with 30ml of dichloroethane, co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com