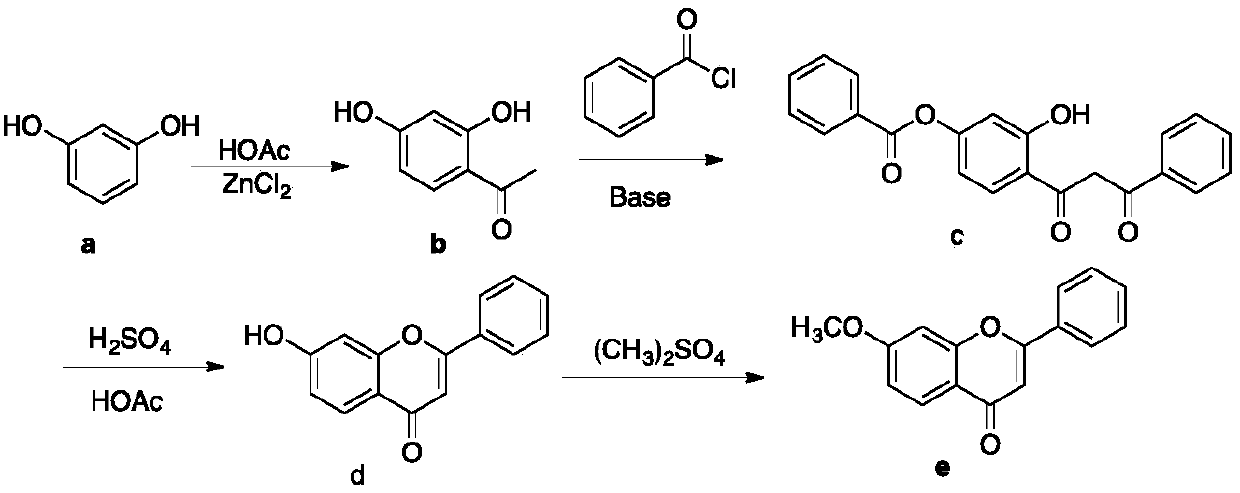
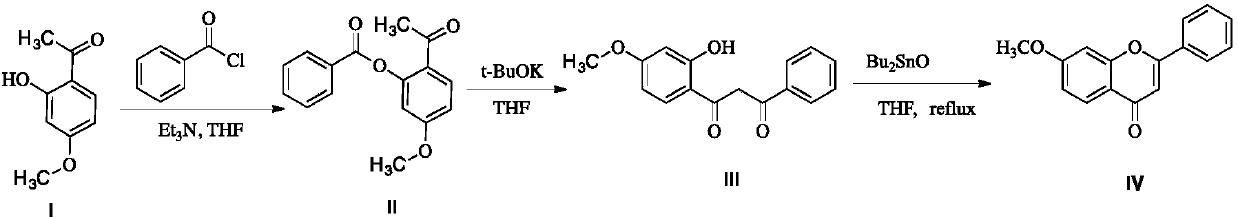
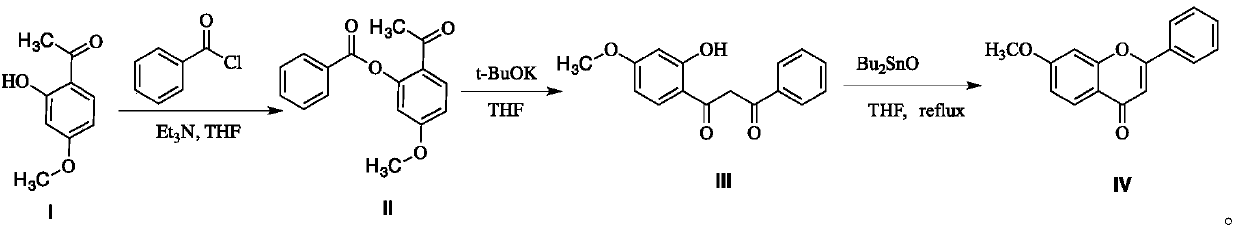
A kind of preparation method of 7-methoxyflavone
A technology of methoxyflavone and benzoyl chloride, which is applied in the field of preparation of 7-methoxyflavone, can solve the problems of reduced product yield, complicated preparation process, loss, etc., and achieves simple operation, easy industrial production, and short route Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Add 16.6 grams (0.1mol) of paeonol, 15.2 grams (0.15mol) of triethylamine and 50 milliliters of tetrahydrofuran (THF) in a 250 mL three-neck round bottom flask, and dissolve 14.7 grams (0.105 mol) of benzoyl chloride in 20 milliliters of tetrahydrofuran at room temperature Slowly add dropwise, control the temperature at about 30 degrees, continue to react for 1 hour after the dropwise addition is completed, remove the triethylamine hydrochloride generated by simple filtration, re-add the filtrate in the original three-necked flask, and add 13.5g (0.12 mol) Potassium tert-butoxide, continue to react for 0.5 hour after adding, add dibutyl tin oxide (accounting for 10% of paeonol mass) after the reaction is completed, and react the reaction mixture for 3 hours in stirring reflux. After the reaction was completed, dibutyltin oxide was removed by filtration while it was hot, and the filtrate was concentrated to obtain the crude product of 7-methoxyflavone, which was recrystal...
Embodiment 2
[0027] Add 16.6 grams (0.1mol) of paeonol, 15.2 grams (0.15mol) of triethylamine and 50 milliliters of tetrahydrofuran in a 250 mL three-neck round bottom flask, and dissolve 25.3 grams of benzoyl chloride (0.18 mol) in 40 milliliters of tetrahydrofuran at room temperature Slowly add dropwise, control the temperature at about 20 degrees, continue to react for 1.5 hours after the dropwise addition, remove the triethylamine hydrochloride generated by simple filtration, re-add the filtrate in the original three-necked flask, add 14.6g (0.13 mol) Potassium tert-butoxide, continue to react for 0.7 hours after the addition, add dibutyl tin oxide (accounting for 20% of paeonol mass) after the reaction, and react the reaction mixture for 12 hours in stirring reflux. After the reaction was completed, dibutyltin oxide was removed by filtration while it was hot, and the filtrate was concentrated to obtain the crude product of 7-methoxyflavone, which was recrystallized from absolute ethano...
Embodiment 3
[0029] Add 16.6 grams (0.1mol) of paeonol, 15.2 grams (0.15mol) of triethylamine and 60 milliliters of tetrahydrofuran in a 250 mL three-neck round bottom flask, and dissolve 19.7 grams (0.14 mol) of benzoyl chloride in 30 milliliters of tetrahydrofuran at room temperature Slowly add dropwise, control the temperature at about 25 degrees, continue to react for 2 hours after the dropwise addition is completed, remove the triethylamine hydrochloride generated by simple filtration, re-add the filtrate to the original three-necked flask, and add 13.5g (0.12 mol) Potassium tert-butoxide, continue to react for 0.7 hours after the addition, add dibutyltin oxide (accounting for 5% of paeonol mass) after the reaction, and react the reaction mixture for 8 hours under stirring reflux. After the reaction was completed, dibutyltin oxide was removed by filtration while it was hot, and the filtrate was concentrated to obtain crude 7-methoxyflavone, which was recrystallized from absolute ethano...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com