Alkaline pectinase mutant with improved secretion property
A pectinase and mutant technology, applied in the field of enzyme engineering, can solve the problems of long fermentation period, high energy consumption for low temperature induction, complicated process, etc., and achieve the effect of improving secretion efficiency and thermodynamic stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Embodiment 1: the acquisition of mutant strain
[0025] Use PCR amplification or chemical synthesis to obtain the alkaline pectinase gene whose amino acid sequence is shown in SEQ ID NO.2, then connect the gene to pET-22b(+), and then transform it into Escherichia coli E.coli BL21 (DE3), screened to obtain the correct transformant.
Embodiment 2
[0026] Embodiment 2: the verification of mutant strain
[0027] Seed culture: Pick the recombinant strain E.coli BL21(DE3) from the plate and inoculate it in LB medium (100 μg·mL -1 ampicillin, 2% glucose), the filling volume is 20mL / 250mL. 37℃, 200r·min -1 Incubate on a shaker for 10 h.
[0028] Shake flask fermentation: the seed solution cultivated for 10 h was inserted into the fermentation medium TB (100 μg·mL) with an inoculum of 3% (V / V) -1 Ampicillin), the filling volume is 20mL / 250mL, 37℃, 200r·min -1 Cultivate to cell concentration OD 600 =0.6, adding a final concentration of 0.04mM IPTG for induction, and induced at 30°C for 48h.
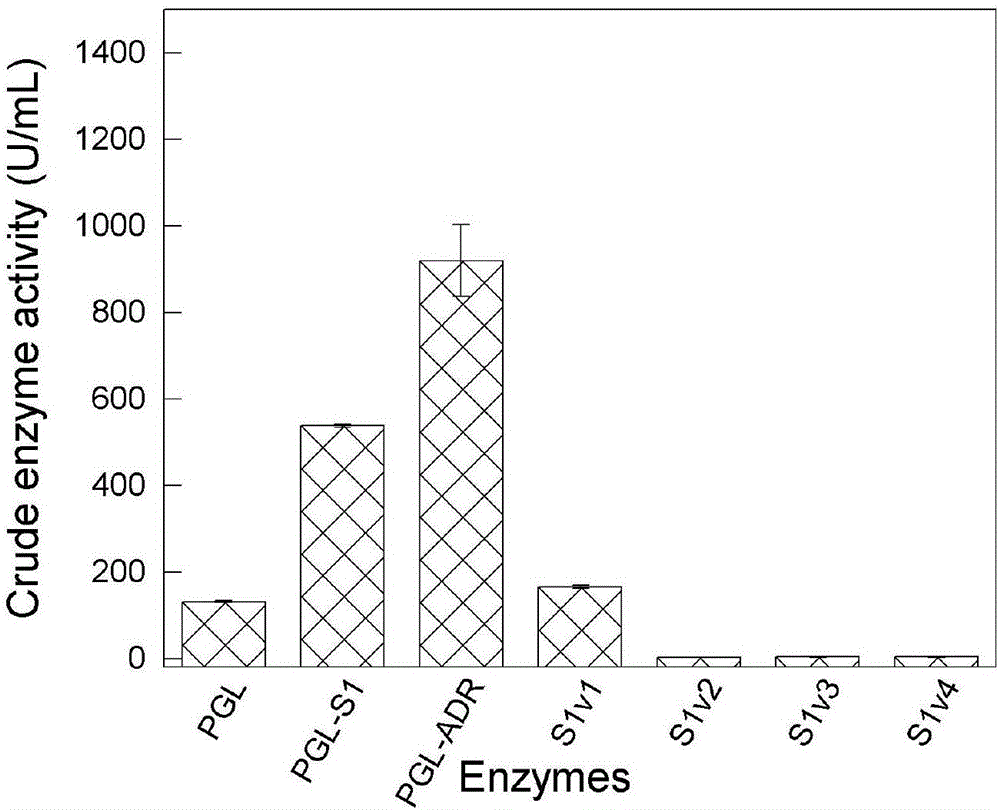
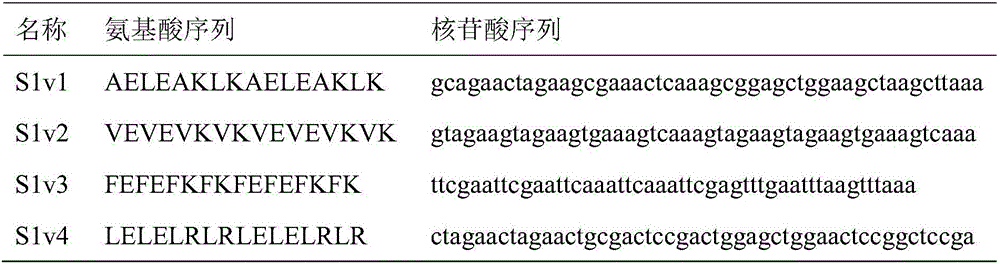
[0029] With the unmutated alkaline pectinase as a contrast, the recombinant bacteria and the control were cultured and fermented according to the above method, and the recombinant bacteria fermentation liquid that had been fermented was centrifuged at 8000r / min for 20min, the supernatant was taken, and the enzyme activity of the super...
Embodiment 3
[0030] Embodiment 3: the purification of alkaline pectinase
[0031] Centrifuge the recombinant bacterial fermentation broth at 8000r / min for 20min, take the supernatant, add ammonium sulfate for gradient salting-out, collect 30-50% ammonium sulfate precipitated part by low-temperature centrifugation, and dissolve the salted-out precipitated enzyme in glycine-sodium hydroxide buffer Solution (pH7.5), dialyzed with 20mmol / L glycine-sodium hydroxide buffer solution for 24h. The supernatant obtained by centrifugation was further separated, purified and desalted by cation exchange chromatography. Dilute the purified alkaline pectinase, measure the enzyme activity according to the above method, and measure the protein concentration with the BSA protein concentration assay kit. The results show that the specific enzyme activity of the mutant PGL-ADR is 448.36±4.73U / mg.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com