Preparation method of herba lysimachiae granules
A technology of desmodium moss and granules, which is applied to medical preparations with non-active ingredients, medical preparations containing active ingredients, and pharmaceutical formulas, etc. It can solve the problems of low content, poor solubility, and difficulty in granulation of chrysanthemum chrysalis granules, etc. problems, to achieve the effects of reduced energy consumption, improved main active ingredients, and strong drying capacity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
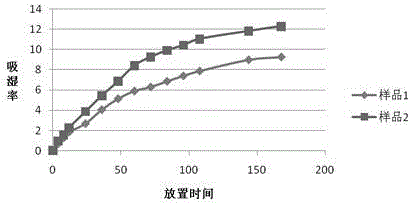
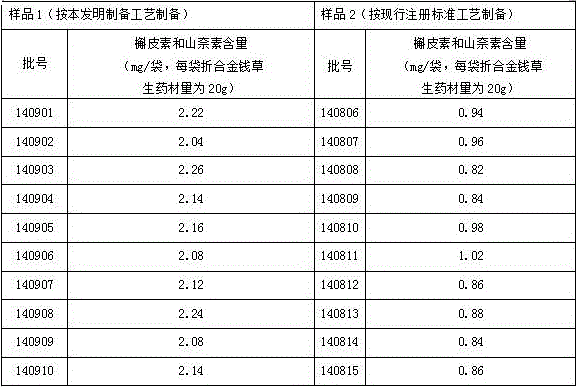
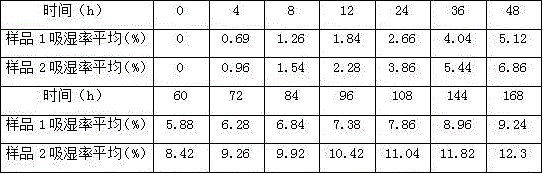
Image
Examples
preparation example Construction
[0023] A preparation method of Desmodium sativa granules, comprising the following steps:
[0024] 1. Desmodium desmodium extract: Desmodium desmodium extract is extracted by forced dynamic circulation. The specific operation method is to take 400 parts by weight of desmodium herbaceous material, add water (90-95 ℃) to force dynamic circulation extraction three times, add 6 times the amount of water for the first time, Forced dynamic circulation extraction for 2 hours, add 4 times the amount of water for the second time, force dynamic circulation extraction for 1 hour, add 2 times the amount of water for the third time, force dynamic circulation extraction for 30 minutes, combine the extracts; let stand for 12 to 24 hours, take the The clear liquid is filtered; the filtrate is concentrated to 75 to 130 parts by weight of extract with a relative density of 1.10 to 1.25 at a temperature of 50 to 55°C;
[0025] 2. Add suitable pharmaceutical excipients and appropriate amount of f...
Embodiment 1
[0029] Desmodium 400g, add water (90-95°C) to extract three times with forced dynamic circulation, add 6 times the amount of water for the first time, and extract with forced dynamic circulation for 2 hours; add 4 times the amount of water for the second time, and extract with forced dynamic circulation for 1 hour; Add 2 times the amount of water, extract by forced dynamic circulation for 30 minutes; combine the extracts, let stand for 12 hours, take the supernatant, filter, and concentrate the filtrate to 75g of extract with a relative density of 1.10 at a temperature of 55°C, and the solid content is 30g; add 0.9g of polyvinylpyrrolidone and 0.6g of aspartame, stir well with a high-speed mixer, obtain a dry extract through a vacuum low-temperature continuous dryer (vacuum degree -0.09MPa, material temperature 60°C), and crush; add 75% Ethanol, granulated, passed through a 12-mesh sieve, made into wet granules, and dried at 65°C to obtain 31.2g of granules, which were divided ...
Embodiment 2
[0031]Desmodium 4000g, add water (90-95 ℃) to force dynamic circulation extraction three times, add 6 times of water for the first time, and force dynamic circulation to extract for 2 hours; add 4 times of water for the second time, and force dynamic circulation to extract for 1 hour; third time Add 2 times the amount of water, extract by forced dynamic circulation for 30 minutes; combine the extracts, let stand for 12 hours, take the supernatant, filter, and concentrate the filtrate to 1298g extract with a relative density of 1.10 at a temperature of 55°C, and the solid content is 498g; add 0.5g sodium carboxymethyl cellulose and 2.5g stevioside, stir evenly with a high-speed mixer, and obtain a dry extract through a vacuum low-temperature continuous dryer (vacuum degree -0.09MPa, material temperature 60°C, crushing particle size 80 mesh) Powder; add 75% ethanol, granulate, pass through a 12-mesh sieve to make wet granules, and dry at 65°C to obtain 500g of granules, which are...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com