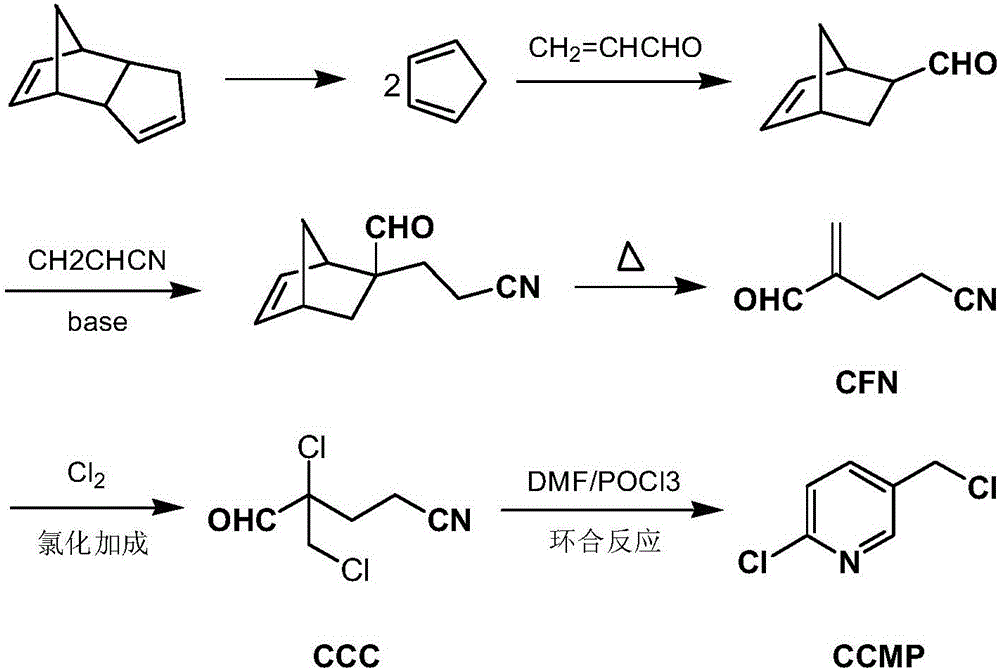
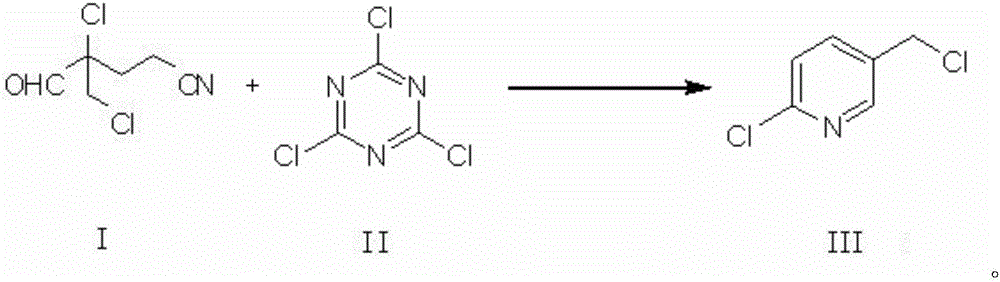
Preparation method of 2-chloro-5-chloromethylpyridine
A technology of chloromethylpyridine and chloromethyl, which is applied in the field of preparation of 2-chloro-5-chloromethylpyridine, can solve problems such as large amount of wastewater, abnormal marine ecology, and high cost of solvents, and achieve economic benefits, The effect of high product quality and low production cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0051] One aspect of the present invention provides a method for preparing 2-chloro-5-chloromethylpyridine, the preparation method of 2-chloro-5-chloromethylpyridine may include: 2-chloro-2-chloromethyl- 4-cyanobutyraldehyde (compound of formula I, referred to as CCC) reacts with cyanuric chloride (compound of formula II, referred to as TCT) to prepare 2-chloro-5-chloromethylpyridine (compound of formula III, CCMP). Said reaction can be ring closure reaction, and reaction equation is as follows:
[0052]
[0053]In the preparation method of 2-chloro-5-chloromethylpyridine provided by the present invention, the reaction can be carried out in the presence of a solvent, and the solvent can be an organic solvent. For example, the organic solvent that can be used includes but is not limited to alkanes One or a combination of solvents, cycloalkane solvents, halogenated alkane solvents, halogenated cycloalkane solvents, aromatic hydrocarbon solvents, halogenated aromatic hydrocarb...
Embodiment 1-1
[0077] Preparation of 2-chloro-5-chloromethylpyridine:
[0078]Get compound CCC 0.1mol, be dissolved in 20 milliliters of ethylene dichloride, oil bath is heated to 80 ℃, add 2g catalyst triethylamine, dropwise add the dichloroethane of 20 milliliters containing 7.37g (0.04mol) cyanuric chloride Solution, keep the temperature between 80-85°C, add dropwise for 2 hours, keep warm at 80°C for 30 minutes, then cool down to 50°C, add 10 ml of water, then add 10.7g of 30% NaOH aqueous solution dropwise at 20-50°C, water Phase pH value is about 8.0-10.0, start to filter, filter cake is rinsed with 10 milliliters of dichloroethanes, the separated filtrate stands for stratification, the lower layer is the dichloroethane phase, desolvation, obtains 17.1g brown solid, GC The standard detection contained CCMP 74.6%, and the yield was 78.7%.
Embodiment 1-2
[0080] Preparation of 2-chloro-5-chloromethylpyridine:
[0081] Take 0.1mol of compound CCC, dissolve it in 20ml of cyclohexane, heat the oil bath to 80°C, add 1g of catalyst N,N-dimethylaniline, dropwise add 20ml of cyanuric chloride containing 11.06g (0.06mol) Cyclohexane solution, keep the temperature between 75-80°C, add dropwise for 2h, keep warm at 80°C for 30 minutes, then cool down to 50°C, add 10ml of water, then add 10.7g of 30% NaOH aqueous solution dropwise at 20-50°C , the pH value of the aqueous phase is about 8.0-10.0, start to filter, rinse the filter cake with 10 milliliters of cyclohexane, and separate the filtrate to stand for stratification. The standard detection contained CCMP 75.6%, and the yield was 81.4%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com