Process system and method for preparing carnosine
A process system and carnosine technology, applied in the field of preparing carnosine, can solve the problems of high solvent consumption, unfavorable industrialization, environmental pollution, etc., and achieve the effects of high reaction generation rate, favorable industrialization, and low energy consumption
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
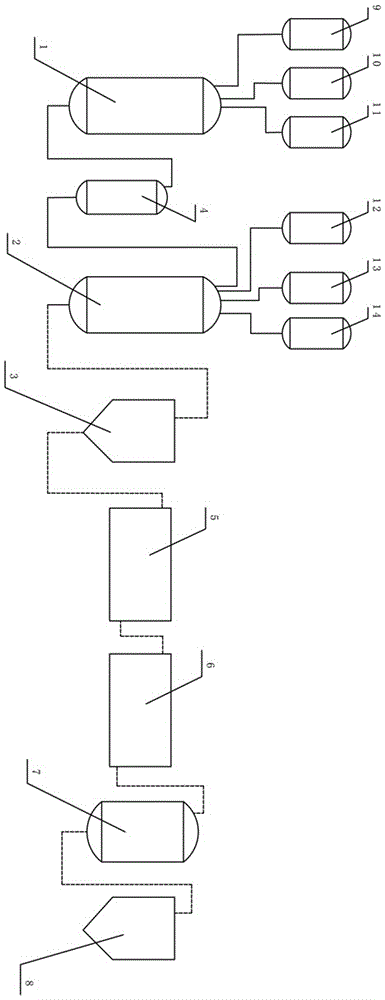
[0025] Such as figure 1 , a process system for preparing carnosine, comprising a first reactor (1), a second reactor (2), a first centrifuge (3), ultrafiltration equipment (5), nanofiltration equipment (6), crystallization tank (7), second centrifuge (8); also includes methanol storage tank (9), thionyl chloride storage tank (10), β-alanine storage tank (11), the methanol storage tank (9 ), the thionyl chloride storage tank (10), and the β-alanine storage tank (11) are located upstream of the first reaction kettle (1), and are respectively connected to the first reaction kettle (1) by pipelines; Including L-histidine storage tank (12), deionized water storage tank (13), alkaline solution storage tank (14); said L-histidine storage tank (12), deionized water storage tank (13 ), the alkaline solution storage tank (14) is located upstream of the second reaction kettle (2), and is connected to the second reaction kettle (2) respectively; A tank (4), the β-alanine methyl ester hy...
Embodiment 2
[0038] Embodiment 2: Synthesis of β-alanine methyl ester hydrochloride
[0039] 1. Accurately weigh 400g of methanol and 220g of thionyl chloride, control the temperature at 0-5°C, slowly add thionyl chloride into methanol dropwise, after the addition is complete, add 160g of β-alanine, and keep warm at 30-35°C Stir for 2h;
[0040] 2. At the end of the heat preservation, depressurize at 35-40°C, vacuum degree ≤ -0.09MPa, concentrate until no fraction flows out, and obtain 245g of β-alanine methyl ester hydrochloride;
[0041] 3. Add 20g of L-histidine, 400g of deionized water, and 25g of β-alanine methyl ester hydrochloride into the reaction bottle, and stir evenly;
[0042] 4. At 25-30°C, adjust the pH to 7.0-7.5 with 30% sodium hydroxide;
[0043] 5. Stir and react at 25-30°C for 4.0 hours. During the reaction, use 5% NaOH solution to control the pH value of the reaction system at 7.0-7.5.
[0044] 6. After the reaction is completed, the content of carnosine in the react...
Embodiment 3
[0048] Embodiment 3: Enzyme reaction embodiment
[0049] 1. Accurately weigh 500g of methanol and 280g of thionyl chloride, control the temperature at 10-15°C, slowly add thionyl chloride into methanol dropwise, after the addition is complete, add 200g of β-alanine, and keep warm at 35-40°C Stir for 4h;
[0050]2. At the end of the heat preservation, depressurize at 45-50°C, vacuum degree ≤ -0.09MPa, concentrate until no fraction flows out, and obtain 307g of β-alanine methyl ester hydrochloride;
[0051] 3. Add 30g of L-histidine, 600g of deionized water, and 37.5g of β-alanine methyl ester hydrochloride into the reaction bottle, and stir evenly;
[0052] 4. At 20-25°C, adjust the pH to 7.5-8.0 with 20% ammonia water;
[0053] 5. Stir and react at 20-25°C for 3.0 hours. During the reaction, use 20% ammonia solution to control the pH value of the reaction system at 7.5-8.0.
[0054] 6. After the reaction is completed, the content of carnosine in the reaction solution is 6.3...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com