A kind of preparation method of polysarcosine block copolymer
A technology of block copolymers and polysarcosine, which is applied in the field of preparation of amphiphilic polyvinyl block polysarcosine block copolymers, and can solve problems such as amphiphilic block copolymers that have not been studied
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
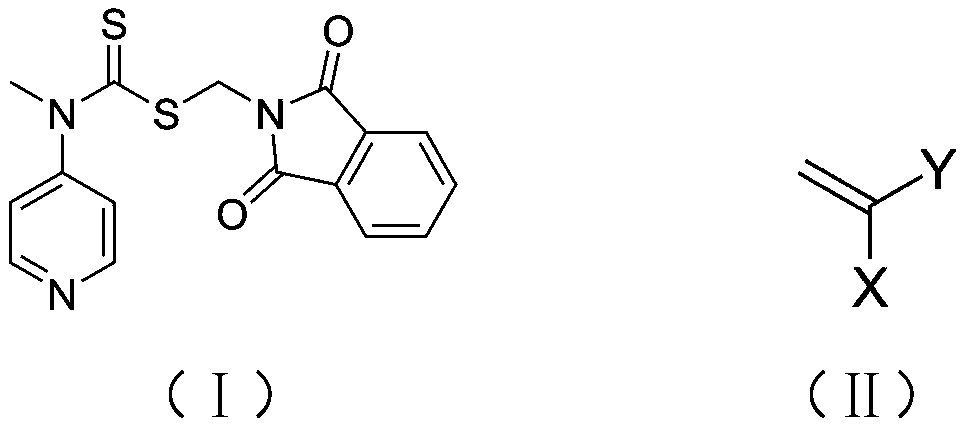
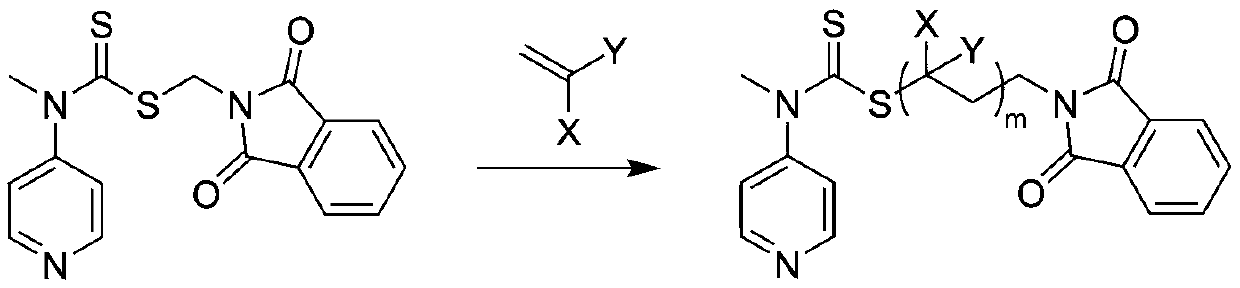
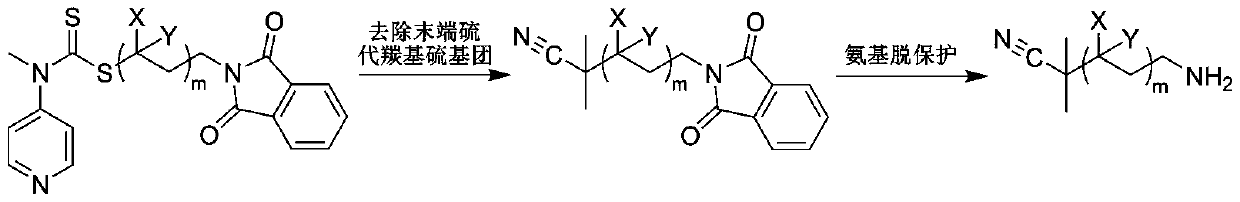
[0041] In the reaction flask, add the RAFT reagent (0.342g, 1mmol) shown in structure (I), N-ethylacrylamide (1.983g, 20mmol), azobisisobutyronitrile (0.033g, 0.2mmol), 8mL of anhydrous benzene was subjected to three freeze-pump-thaw cycles to remove the oxygen in the reaction system, sealed in a nitrogen atmosphere, and reacted at 60°C for 2h, with a conversion rate of 97%. Mix tri-n-butyl tin hydrogen (11.6g, 40mmol), azobisisobutyronitrile (0.033g, 0.2mmol), and 1mL of anhydrous benzene to form a solution, add it to the reaction flask with a syringe, and react at 70°C for 3h to remove the polymer The terminal thiocarbonylthio group. Hydrazine hydrate (1 g, 20 mmol) was added to the reaction solution, and reacted at 60° C. for 1 h to release the terminal amino group of the polymer. Under the condition of nitrogen flow in the reaction system, N-methyl substituted glycine-N-carboxy anhydride (2.3g, 20mmol) was added to the reaction solution, and the molar ratio of monomer to ...
Embodiment 2
[0043] In the reaction flask, add the RAFT reagent (0.342g, 1mmol) shown in structure as (I), N-propyl acrylamide (5.658g, 50mmol), azobisisobutyronitrile (0.033g, 0.2mmol), 20mL of anhydrous acetonitrile was subjected to three freeze-pump-thaw cycles to remove oxygen in the reaction system, sealed under a nitrogen atmosphere, and reacted at 70°C for 2 hours, with a conversion rate of 95%. Excess azobisisobutyronitrile (3.3g, 20mmol) and 1mL of anhydrous acetonitrile were mixed into a solution, added into the reaction flask with a syringe, and reacted at 80°C for 6h to remove the thiocarbonylthio group at the end of the polymer. Hydrazine hydrate (1 g, 20 mmol) was added to the reaction solution, and reacted at 60° C. for 1 h to release the terminal amino group of the polymer. Under the nitrogen flow condition of the reaction system, N-methyl substituted glycine-N-carboxy anhydride (5.755g, 50mmol) was added to the reaction solution, and the molar ratio of monomer to macromolecu...
Embodiment 3
[0045] In the reaction flask, add the RAFT reagent (0.342g, 1mmol) shown in structure (I), N-propylacrylamide (16.974g, 150mmol), azobisisobutyronitrile (0.033g, 0.2mmol), 60mL of anhydrous toluene was subjected to three freeze-pump-thaw cycles to remove oxygen in the reaction system, sealed in a nitrogen atmosphere, and reacted at 70°C for 4h, with a conversion rate of 96%. Excess azobisisobutyronitrile (3.3g, 20mmol) and 1mL of anhydrous toluene were mixed into a solution, and added into the reaction flask with a syringe, and reacted at 80°C for 8h to remove the thiocarbonylthio group at the end of the polymer. Hydrazine hydrate (1 g, 20 mmol) was added to the reaction liquid, and reacted at 60° C. for 2 h to release the terminal amino group of the polymer. Under the nitrogen flow condition of the reaction system, N-methyl substituted glycine-N-carboxy anhydride (5.755g, 50mmol) was added to the reaction solution, and the molar ratio of monomer to macromolecular initiator wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com