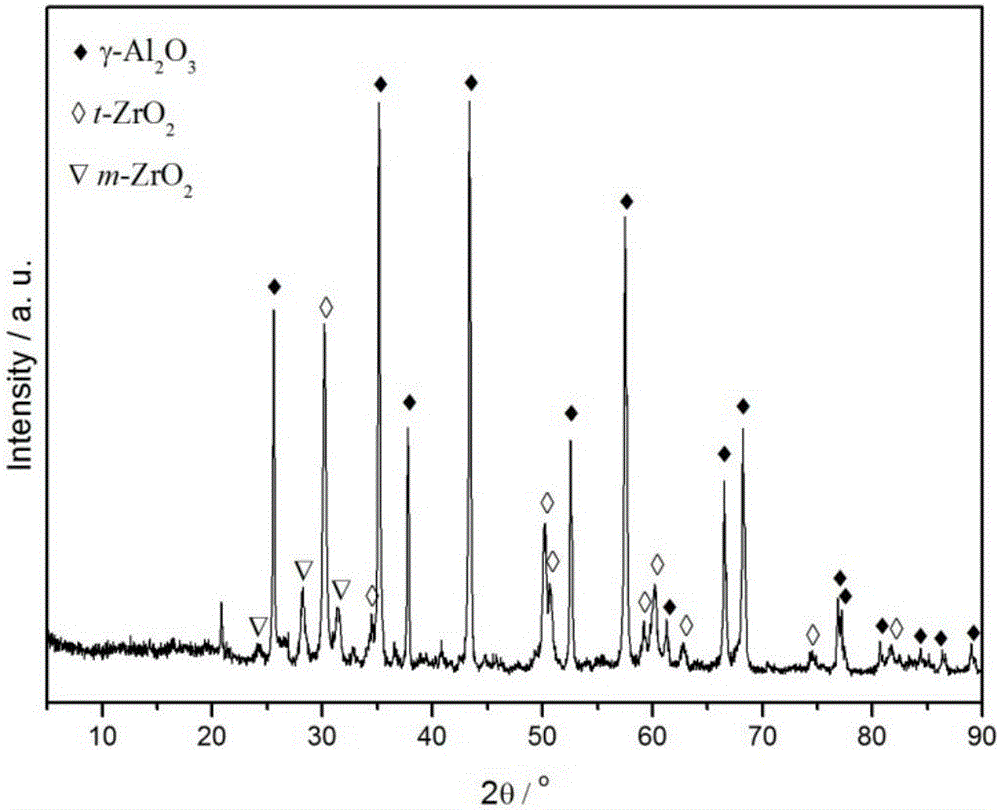
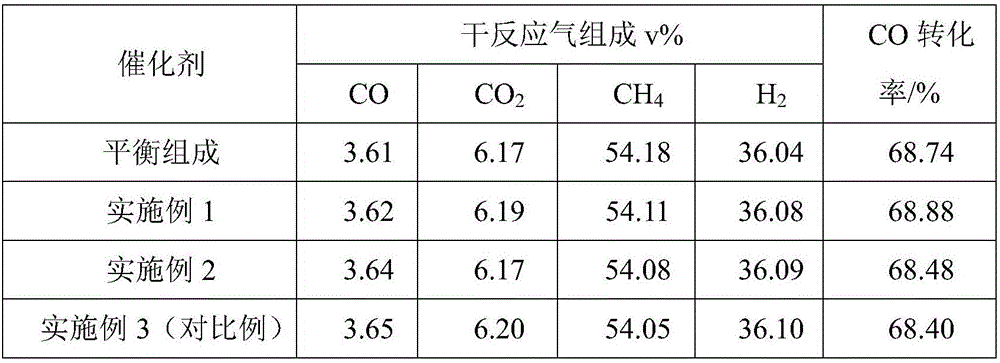
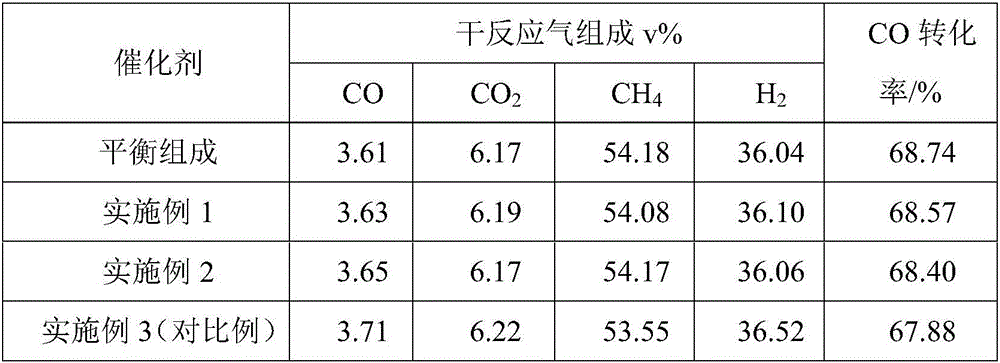
Zirconium-aluminum composite oxide-loaded nickel-base methanation catalyst and preparation method thereof
A methanation catalyst and composite oxide technology, applied in the petroleum industry, gas fuel, fuel, etc., can solve the problems that restrict the development of syngas methanation, carbon deposition deactivation, high-temperature sintering deactivation, etc., and achieve small particle size, anti-corrosion Good carbon properties and good low temperature activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0028] 75.00g Al 2 o 3 and 25.00 g ZrO 2 Mix, use a ball mill to mix evenly, then add 15.00g of carbon black pore-forming agent and mix evenly, pass through a 200-mesh sieve, add 3mL of water, mix evenly, and use a ring press machine to press Φ6×6mm into a columnar shape. The columnar mixture was dried in an oven at 120°C for 8 hours, and then placed in a muffle furnace for calcination at 950°C for 5 hours to obtain a zirconium-aluminum composite oxide support.
[0029] The above support was added to an excess of 1.65 g / mL Ni(NO 3 ) 2 ·6H 2 O and 0.18g / mLLa(NO 3 ) 3 ·6H 2 Immerse in a mixed solution of O for 0.5h, take out the solid after immersion, dry in an oven at 120°C for 8h, and then place it in a muffle furnace for calcination at 400°C for 5h to obtain a nickel-based methanation catalyst supported by zirconium-aluminum composite oxide.
Embodiment 2
[0031] 43.75g Al 2 o 3 and 46.25 g ZrO 2 Mix, use a ball mill to mix evenly, then add 20.00g of carbon black pore-forming agent and mix evenly, pass through a 200-mesh sieve, add 3mL of water, mix evenly, and press it into a columnar shape with a ring press machine according to Φ6×6mm. The columnar mixture was dried in an oven at 100° C. for 10 h, and then placed in a muffle furnace for calcination at 1000° C. for 5 h to obtain a zirconium-aluminum composite oxide support.
[0032] The above support was added to an excess of 1.68 g / mL Ni(NO 3 ) 2 ·6H 2 O and 0.17g / mLCe(NO 3 ) 4 ·6H 2 Immerse in a mixed solution of O for 1 hour, take out the solid after immersion, dry in an oven at 100°C for 10 hours, and then bake in a muffle furnace at 400°C for 5 hours to obtain a nickel-based methanation catalyst supported by zirconium-aluminum composite oxide.
Embodiment 3
[0034] This example is a comparative example. Compared with Example 1, the catalyst carrier in this example does not add pore-forming agent carbon black, and the rest of the conditions are the same as Example 1.
[0035] 43.75g Al 2 o 3 and 46.25 g ZrO 2 Mix, use a ball mill to mix evenly, pass through a 200-mesh sieve, add 3mL of water, mix evenly, and use a ring press machine to press Φ6×6mm to form a columnar shape. The columnar mixture was dried in an oven at 100° C. for 10 h, and then placed in a muffle furnace for calcination at 1000° C. for 5 h to obtain the comparative carrier.
[0036] The above comparative support was added to an excess of 1.68 g / mL Ni(NO 3 ) 2 ·6H 2 O and 0.17g / mLCe(NO 3 ) 4 ·6H 2 Immerse in a mixed solution of O for 1 hour, take out the solid after soaking, dry in an oven at 100°C for 10 hours, and then place it in a muffle furnace for calcination at 400°C for 5 hours to obtain a comparative catalyst without carbon black pore-forming agent....
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com