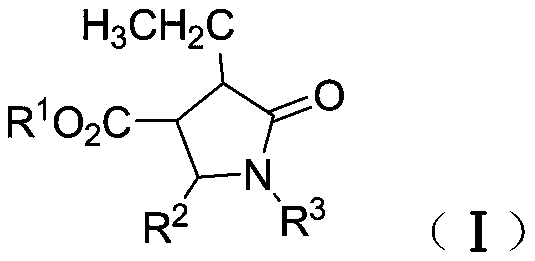
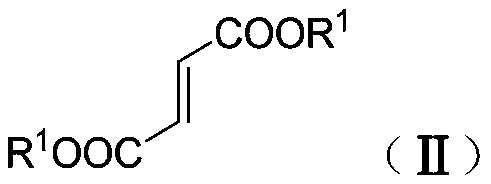
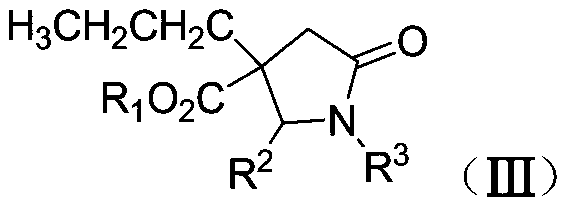
Synthetic method of β-ester group-γ-butyrolactam and γ-ester group-δ-valerolactam
A technique for synthesizing butyrolactam, which is applied in the chemical industry, can solve problems such as many reaction steps, low yield, and complicated treatment, and achieve the effects of mild reaction conditions, wide application range, and easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Under nitrogen protection, dimethyl fumarate (130mg, 0.90mmol), benzaldehyde phenylimine (109mg, 0.60mmol), tetrahydrofuran (1.0mL) and toluene (1.0mL) were added to a dry reaction flask and stirred Dissolve, add ZnEt to the above solution under stirring 2 (1.2mL, 1.2mmol), stirred at room temperature for 6h, then added saturated NH 4 Cl aqueous solution (2mL), and continue to stir for 5min, then add dilute hydrochloric acid (2N, 2mL), and continue to stir for 5min, separate the phases, extract the aqueous phase (3×10mL) with dichloromethane, and pass the combined organic phase over saturated salt Washed with water, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain white solid and colorless oily liquid N-phenyl-α-ethyl-β-methoxyacyl-γ-phenyl-γ- Mixture of butyrolactam isomers (isomer ratio 12.4:1) (193 mg, >99% yield).
Embodiment 2
[0044] Under nitrogen protection, dimethyl fumarate (130 mg, 0.90 mmol), p-methoxybenzaldehyde phenylimine (127 mg, 0.60 mmol), tetrahydrofuran (1.0 mL) and toluene ( 1.0mL) was stirred and dissolved, and ZnEt was added to the above solution under stirring 2 (1.2mL, 1.2mmol), stirred at room temperature for 8h, then added saturated NH 4 Cl aqueous solution (2mL), and continue to stir for 5min, then add dilute hydrochloric acid (2N, 2mL, 2mL), and continue to stir for 5min, separate the phases, extract the aqueous phase (3×10mL) with dichloromethane, and combine the organic phase through Washed with saturated brine, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain a colorless oily liquid α-ethyl-β-methoxyacyl-N-phenyl-γ-p-methoxyphenyl - Mixture of isomers of γ-butyrolactam (isomer ratio 10:1) (212 mg, >99.9% yield).
Embodiment 3
[0046] Under nitrogen protection, dimethyl fumarate (130mg, 0.90mmol), benzaldehyde phenylimine (109mg, 0.60mmol), tetrahydrofuran (1.0mL) and toluene (1.0mL) were added to a dry reaction flask and stirred Dissolve, add ZnEt to the above solution under stirring at -28°C 2 (1.2mL, 1.2mmol), stirred at -28°C for 4h, reacted at room temperature, stirred at room temperature for 4h, then added saturated NH 4 Cl aqueous solution (2mL), and continue to stir for 5min, then add dilute hydrochloric acid (2N, 2mL), and continue to stir for 5min, separate the phases, extract the aqueous phase (3×10mL) with dichloromethane, and pass the combined organic phase over saturated salt Washed with water, dried over anhydrous sodium sulfate, concentrated, and separated by column chromatography to obtain white solid and colorless oily liquid N-phenyl-α-ethyl-β-methoxyacyl-γ-phenyl-γ- Mixture of butyrolactam isomers (isomer ratio 8.9:1) (194 mg, >99.9% yield).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com