Berberine drug as well as preparation method and application thereof
A diabetes drug and reaction technology, applied in the field of medicine, can solve problems such as high risk, many side effects, and increased impurities, and achieve the effect of overcoming poor solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] Embodiment 1: the preparation of intermediate 1
[0049]
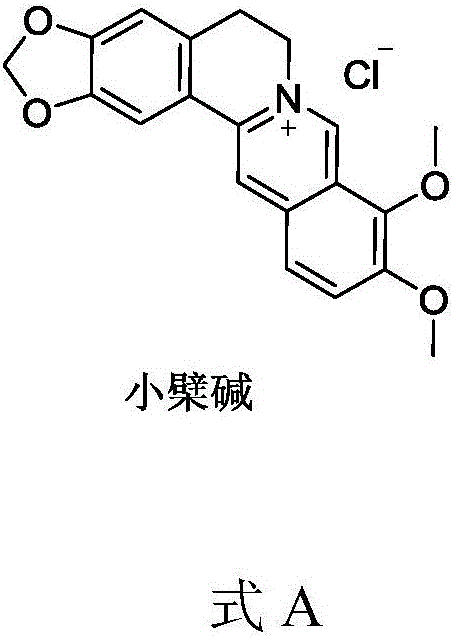
[0050] Suspend 2 g of berberine (purchased from Sichuan Weikeqi Biotechnology Co., Ltd.) in 50 ml of sodium hydroxide solution (concentration 0.1 mol / L), add 1 times the amount of succinic anhydride at 10 degrees Celsius, and stir for 1 hour after adding, Add 0.5 mol / L hydrochloric acid solution dropwise in an ice bath to adjust the pH to about 6.5, collect the solid by filtration, and dry under reduced pressure to obtain the product intermediate 1, which is directly used in the next reaction. Yield 65%. LC-Ms: ESI: 423.3 (M+H).
Embodiment 2
[0051] Embodiment 2: the preparation of compound BT-1 of the present invention
[0052]
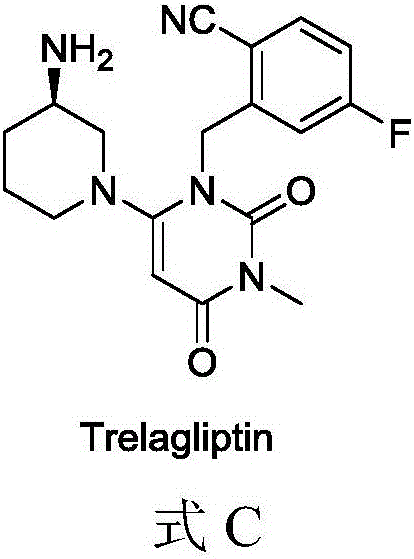
[0053] Suspend 2 g of the intermediate 1 obtained in Example 1 in 50 ml of anhydrous tetrahydrofuran, add 1 times the amount of trexagliptin, add 1.2 times the amount of triethylamine, add 1 times the amount of HATU (purchased from Jill Biochemical) in an ice bath, and naturally Raise the temperature to 20°C and stir for 2 hours.
[0054] Add 100 ml each of dichloromethane and water, separate the organic layer, wash once with water, dry, and concentrate. The crude product was subjected to silica gel column chromatography (methanol:dichloromethane=20:1) to obtain product BT-1 with a yield of 52%.
[0055] 1 H NMR (400MHz, DMSO-d 6 )δ8.68(s,1H),8.50(d,J=8.2Hz,1H),7.84-8.46(m,9H),5.8(s,2H),4.2(s,1H),3.32-3.86(m , 11H), 2.52(t, 2H), 2.41-2.51(m, 8H), 2.22(t, 2H), 1.82-1.91(m, 4H). LC-Ms: ESI: 762.3 (M+H).
Embodiment 3
[0056] Embodiment 3: drug solubility experiment test
[0057] Test method: Weigh 100 mg of the drug to be tested (the reference substance berberine hydrochloride, purchased from Shanghai Yuanye Biotechnology Co., Ltd.), add 1 ml of pure water at 25 degrees Celsius, and shake for 30 seconds every 5 minutes. To dissolve, add 0.1ml of pure water each time, and shake for 30 seconds at intervals of 2 minutes until the drug is completely dissolved.
[0058] The experiment was repeated 3 times for each drug, and the results are shown in Table 1.
[0059] Table 1 Solubility test results
[0060]
[0061]
[0062] Take the average value of the test results of 3 solubility experiments:
[0063] The amount of water to dissolve 50 mg of berberine hydrochloride is (24.9+25.2+25.2) / 3=25.1 (ml)
[0064] The amount of water to dissolve 50mgBT-1 is (4.7+4.8+4.9) / 3=4.8(ml)
[0065] The above experimental results show that the water solubility of the medicine of the present invention i...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com