Efficient preparation method of high-purity lithium oxalyldifluoroborate (LiODFB)
A high-purity technology of lithium difluorooxalate borate, applied in chemical instruments and methods, compounds containing elements of group 3/13 of the periodic table, organic chemistry, etc., can solve the problem of low yield of insoluble impurities, low product purity and cost High cost and other problems, to achieve the effect of easy industrial production, simple preparation method, and reduce the amount of production
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
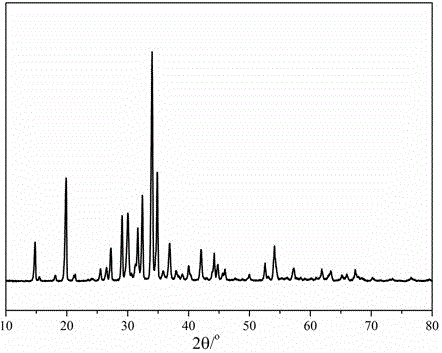
[0021] First weigh 50g Li 2 CO 3 and 103g H 2 C 2 o 4 2H 2 O was used as a raw material to synthesize a mixed crystal A containing Li ions, oxalate and hydrogen oxalate, and its XRD pattern was as follows figure 1 As shown, the phase analysis of mixed crystal A mainly contains Li 2 C 2 o 4 and LiHC 2 o 4 The two phase structures correspond to standard card numbers: 24-0646 and 49-1209 respectively. figure 2The topography photo of the mixed crystal A after crystallization is shown, which has a regular needle-like structure. The mixed crystals A were added to a glass vessel containing the solvent dimethyl carbonate, followed by the addition of 230 g of boron trifluoride dimethyl carbonate complex, 66 g of anhydrous oxalic acid and 64 g of AlCl 3 , magnetically stirred for 30 minutes to obtain milky solution B; filter out the insoluble matter to obtain a clear and transparent solution C; remove the solvent from solution C at 120°C and -0.1MPa to obtain LiODFB product,...
Embodiment 2
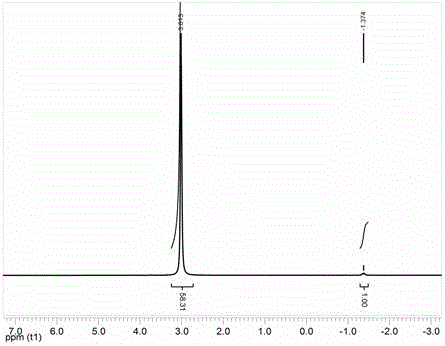
[0023] First weigh 110g Li 2 CO 3 and 243g H 2 C 2 o 4 2H 2 O was used as raw material to synthesize mixed crystal A containing Li ions, oxalate and hydrogen oxalate; the mixed crystal A was added to a polytetrafluoroethylene container containing diethyl carbonate solvent, followed by adding 496 g of boron trifluoride carbonate Diethyl ester complex, 152 g anhydrous oxalic acid and 142 g SiCl 4 , and magnetically stirred for 30 minutes to obtain milky solution B; filter out insoluble matter to obtain clear and transparent solution C; remove the solvent from solution C at 100 °C and -0.05MPa to obtain LiODFB product, and its purity was calculated to be 99% by NMR test. After one recrystallization from the solvent diethyl carbonate, a high-purity LiODFB product was obtained, and its purity was calculated to be over 99.9% through NMR testing. Moisture testing was carried out by a Karl Fischer moisture tester, and the moisture content was measured to be 108 ppm.
Embodiment 3
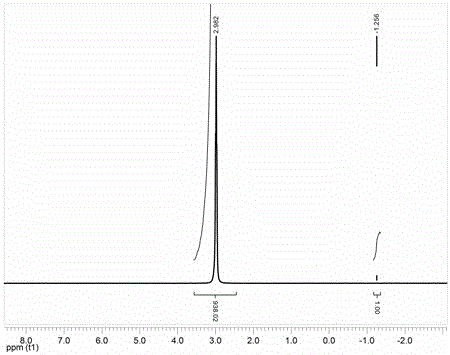
[0025] First weigh 100g Li 2 CO 3 and 346g H 2 C 2 o 4 2H 2 O is raw material, synthesizes the mixed crystal A that comprises Li ion, oxalate and hydrogen oxalate; Add mixed crystal A to the stainless steel airtight container that tetrahydrofuran solvent is housed, then add 347g boron trifluoride tetrahydrofuran complex, 104 g Anhydrous oxalic acid and 161 g TiCl 4 , magnetically stirred for 30 minutes to obtain milky solution B; filter out insoluble matter to obtain clear and transparent solution C; remove the solvent from solution C at -0.07MPa at 105°C to obtain LiODFB product, and its purity was calculated to be 96.8% by NMR test. After one recrystallization through the solvent ethyl acetate, a high-purity LiODFB product was obtained, and its purity was calculated to be over 99.5% through NMR testing. Moisture testing was carried out by a Karl Fischer moisture tester, and the moisture content was measured to be 300 ppm.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com