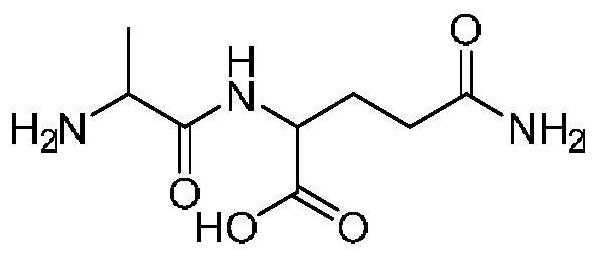
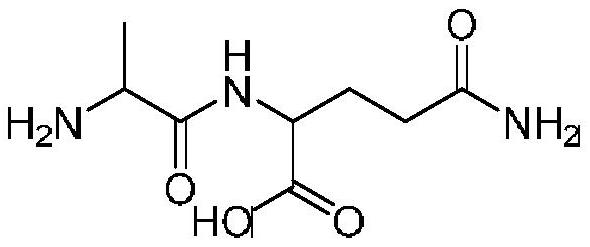
A kind of preparation method of alanyl glutamine
A technology of alanyl glutamine and alanyl succinimide, which is applied in the field of preparation of alanyl glutamine, can solve problems such as difficult removal, short process steps, human harm and the like, and achieves increased yield, Easy to handle and the effect of promoting precipitation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
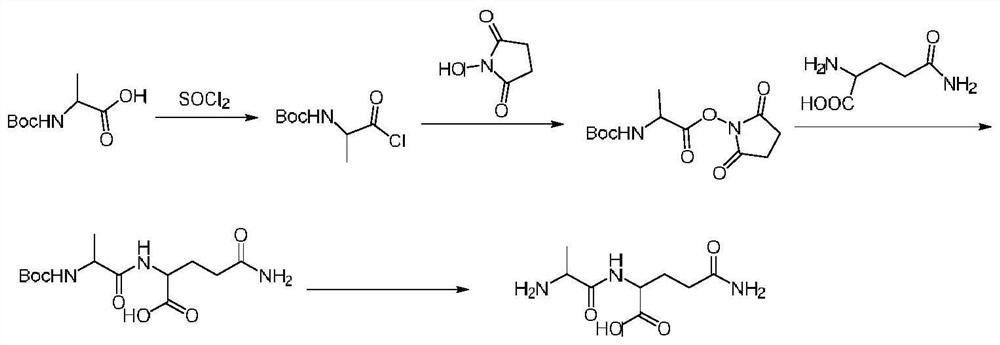
Embodiment 1
[0020] Preparation of chloro-Boc-alanine:
[0021]
[0022] Add Boc-alanine (18.9g, 0.1mol) into the reaction flask, then add thionyl chloride (17.9g, 0.15mol), then add N,N-dimethylformamide (0.7g, 0.01mol ) stirred at 75°C-85°C for 5h, then cooled to 40-50°C, controlled the temperature at 45-55°C and concentrated under reduced pressure until no distillate was obtained, and the obtained oil was directly used in the next reaction.
[0023] Preparation of Boc-alanylsuccinimide:
[0024]
[0025] N-hydroxysuccinimide (13.8g, 0.12mol) was dissolved in 90ml of acetone, then triethylamine (50.5g, 0.5mol) was added, and chloro-Boc-alanine (0.1mol ) was dissolved in 40ml of acetone and added dropwise to the above system. After the addition was completed, it was reacted at room temperature for 4 hours, and then 400g of purified water was added to the reaction system, a large amount of solids would be precipitated, filtered with suction, and the filter cake was rinsed with purif...
Embodiment 2
[0033] Preparation of chloro-Boc-alanine:
[0034]
[0035] Add Boc-alanine (18.9g, 0.1mol) into the reaction flask, then add thionyl chloride (17.9g, 0.15mol), then add N,N-dimethylformamide (0.7g, 0.01mol ) stirred at 75°C-85°C for 5h, then cooled to 40-50°C, controlled the temperature at 45-55°C and concentrated under reduced pressure until no distillate was obtained, and the obtained oil was directly used in the next reaction.
[0036] Preparation of Boc-alanylsuccinimide:
[0037]
[0038] N-hydroxysuccinimide (13.8g, 0.12mol) was dissolved in 90ml of acetone, then triethylamine (50.5g, 0.5mol) was added, and chloro-Boc-alanine (0.1mol ) was dissolved in 40ml of acetone and added dropwise to the above system. After the addition was completed, it was reacted at room temperature for 4 hours, and then 400g of purified water was added to the reaction system, a large amount of solids would be precipitated, filtered with suction, and the filter cake was rinsed with purif...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com