mPEG-SC5K modified matrix metalloproteinase-9 inhibitor polypeptide P2 and application thereof
A matrix metal and protease technology, which is applied in the direction of protease inhibitors, peptide/protein components, peptide preparation methods, etc., can solve the problems of short half-life of polypeptide P2 and high cost of modification, and achieve the effect of prolonging the half-life
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] mPEG-SC 5K Steps to modify P2
[0041] 1. PEGSC 5K Preliminary exploration of modification conditions
[0042] Weigh 32mg mPEG-SC respectively 5K And 10mg P2 (molar ratio is 1.5: 1) when the concentration is 0.05mol / L, 0.1mol / L, 0.15mol / L, 0.2mol / L, 0.25mol / L, prepare the Na of pH7.0 2 HPO 4 -NaH 2 PO 4 buffer, and reacted at 4°C for 3 hours.
[0043] 2. mPEG-SC 5K Optimization of modification conditions
[0044] Weigh 23mg mPEG-SC respectively 5K And 10mg P2 (molar ratio is 1.1:1), 27mg mPEG-SC 5K And 10mg P2 (molar ratio is 1.3:1), 32mg mPEG-SC 5K And 10mg P2 (molar ratio is 1.5:1), 36mg mPEG-SC 5K And 10mg P2 (molar ratio is 1.7:1), 42mg mPEG-SC 5K and 10 mg of P2 (molar ratio: 2.0:1) were placed in 40 mL of prepared PBS buffer solution with pH 8.0-8.5, and reacted at 4° C. for 3-12 h.
[0045] 3. Re-optimization of various PEG modification conditions
[0046] Weigh 128mg mPEG-SC respectively 5K and 40 mg of P2 (molar ratio: 1.5:1) were placed in 40 m...
Embodiment 2
[0063] mPEG-SC 5K Separation and purification steps of modified P2
[0064] 1. Separation
[0065] The sample after the reaction was purified by semi-preparative high-performance liquid phase (HPLC, Shimadzu), and the purification conditions were:
[0066] Semi-preparative chromatographic column: YMC, 250mm×10mm (5μm filler);
[0067] Mobile phase: Phase A is water, phase B is acetonitrile;
[0068] Sample volume: 1mL;
[0069] Flow rate: 2mL / min;
[0070] Detection wavelength: 220nm;
[0071] Elution gradient: see Table 3.
[0072] Table 3 Elution Gradient 2
[0073]
[0074] Collect the product in a centrifuge tube during the eluting process of the target peak.
[0075] 2. Purification
[0076] The product collected by semi-preparative HPLC was pre-frozen overnight in a -80°C low-temperature refrigerator after rotary evaporation, and then lyophilized in a pre-cooled freeze dryer until it was completely white powder by visual inspection (about 48h). Harvest the f...
Embodiment 3
[0090] Enzyme inhibition activity test
[0091] 1. Preparation of buffer solution: Accurately weigh 2.42g Tris, then accurately weigh 1.16g NaCl, add these two samples into 200mL double-distilled water and dissolve them completely, adjust the pH to 7.4 with HCl, and finally accurately Weigh 1.11gCaCl 2 , was added to the solution to dissolve it completely, and the solution was filtered through a 0.22 μm microporous membrane.
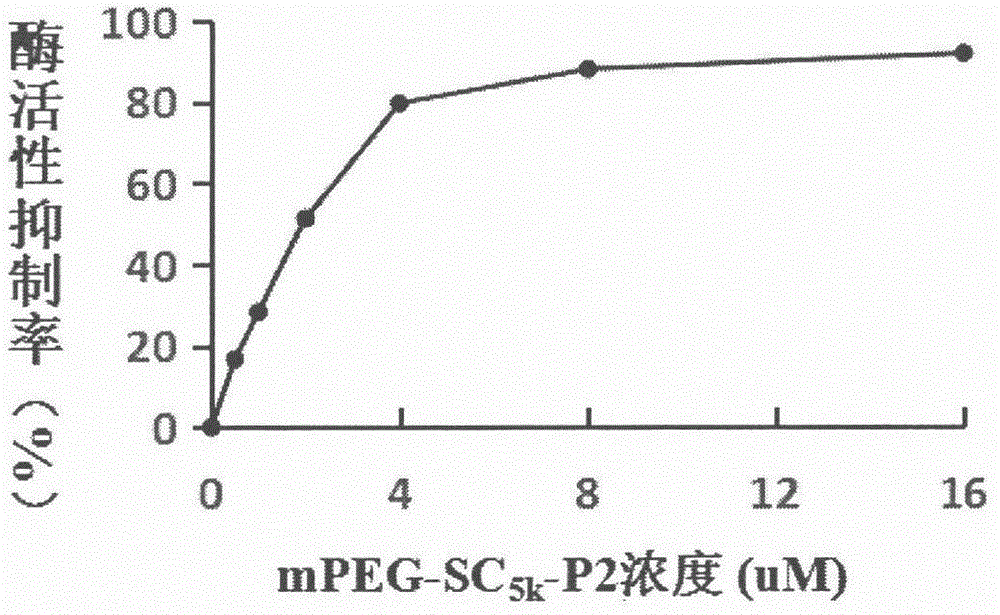
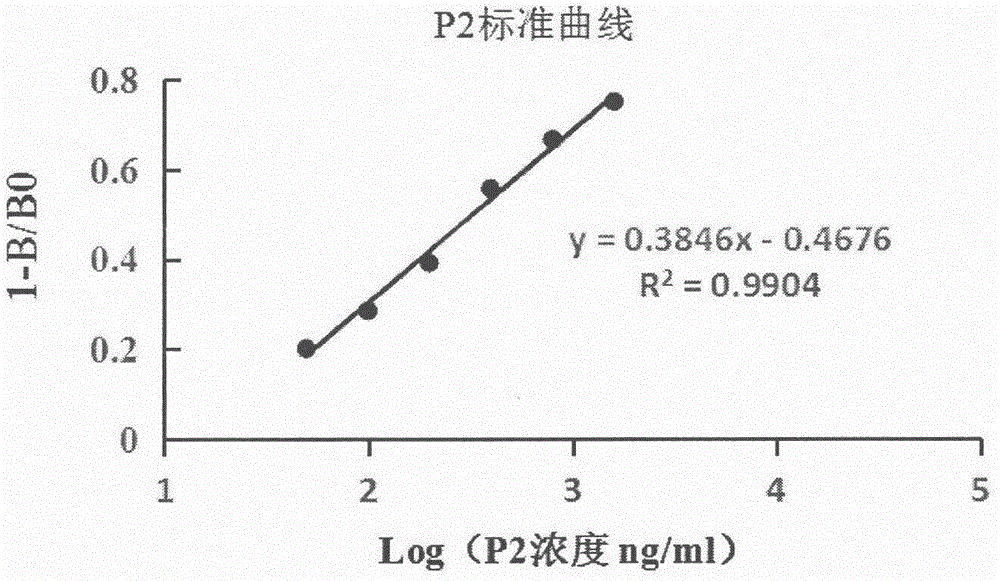
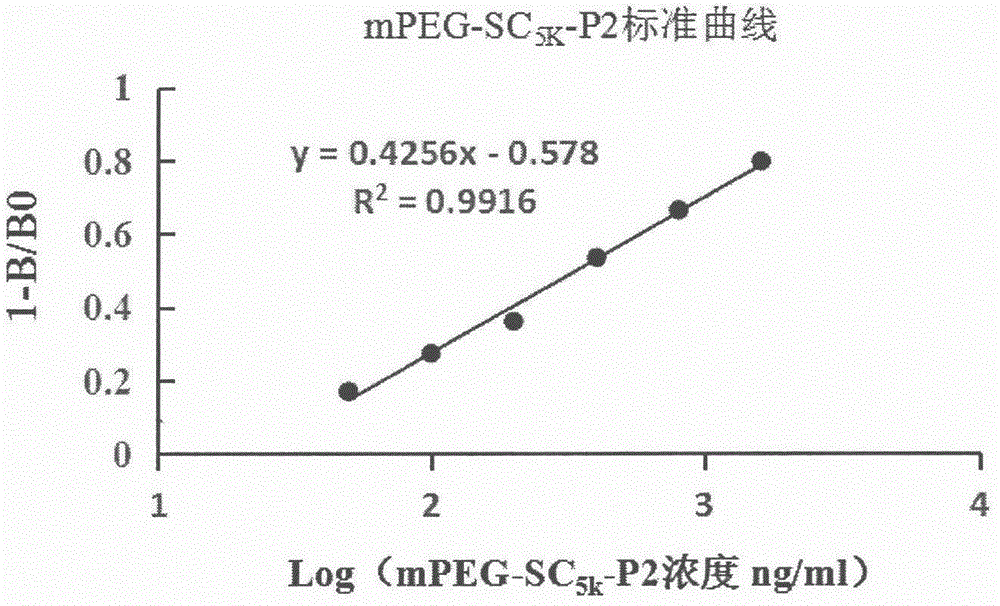
[0092] 2. Detection of mPEG-SC by cleavage of fluorescent substrate (Mca-Pro-Leu-Gly-Leu-Dpa-Ala-Arg-NH2) by matrix metalloproteinase-9 5K -P2 enzyme inhibitory activity, when MMP-9 is cleaved, the fluorescence value of the reaction system shows an increasing trend (set the excitation wavelength to 328nm and the emission wavelength to 392nm), the analysis process should be carried out in a 100μL reaction system at 37°C, This system includes MMP-9 solution, substrate solution, buffer and inhibitor). All the solutions in the reaction system should be ad...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com