Use of hm-3 and platinum, paclitaxel or citabine drugs in the preparation of solid tumor drugs
A paclitaxel, solid tumor technology, applied in antitumor drugs, drug combinations, pharmaceutical formulations, etc., can solve problems such as toxic side effects, complex drug properties, tumor cell drug resistance, complex pathogenesis, etc., to reduce toxic side effects, expand Effects of therapeutic spectrum, significant social value, and market value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
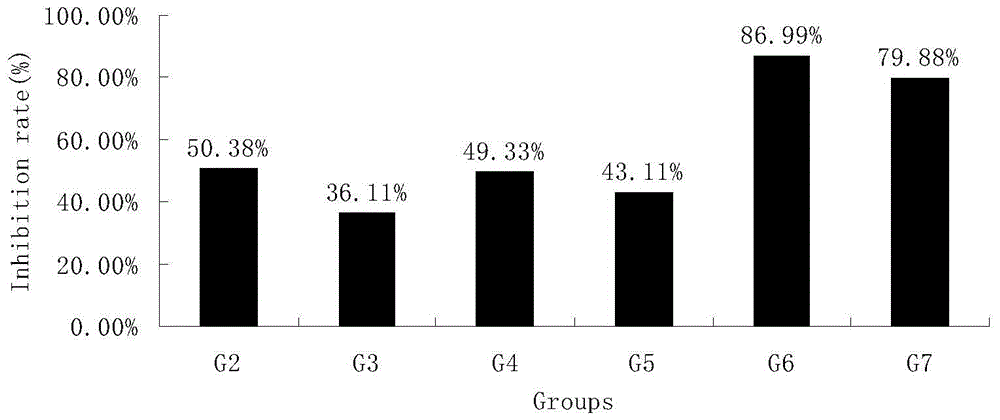
[0044] Example 1 The inhibitory effect of HM-3 combined with oxaliplatin and docetaxel on human lung cancer H460 transplanted tumor in nude mice
[0045] Lung cancer tissues in the vigorous growth stage were taken and ground under sterile conditions, and prepared into 1×10 7 / mL cell suspension, planted in the right armpit of mice with 0.1mL. When the tumor volume reaches 100mm 3 The diameter of the transplanted tumor was measured with a vernier caliper on the left and right, and the anti-tumor effect of the tested animals was dynamically observed. It is administered by tail vein injection or subcutaneous injection, and the dosage regimen is shown in Table 1. Tumor diameters were measured every other day. The administration volume is 0.2ml / only. The negative control was injected with the same volume of saline in the tail vein. After 21 days, the mice were sacrificed, and the tumor mass was removed and weighed. The formula for calculating tumor volume (TV) is:
[0046] T...
Embodiment 2
[0062] Example 2 The inhibitory effect of HM-3 combined with oxaliplatin and Xeloda on transplanted tumors of human liver cancer SMMC-7721 in nude mice
[0063] Liver cancer tissues in vigorous growth phase were taken and ground under sterile conditions, and prepared into 1×10 7 / mL cell suspension, planted in the right armpit of mice with 0.1 mL. Others participate in Example 1.
[0064] The test was divided into 7 groups, HM-3 group with 8 rats in each group, other treatment groups with 10 rats in each group, and control group with 13 rats. See Table 3 for the specific dosage regimen.
[0065] Table 3 Administration settings
[0066]
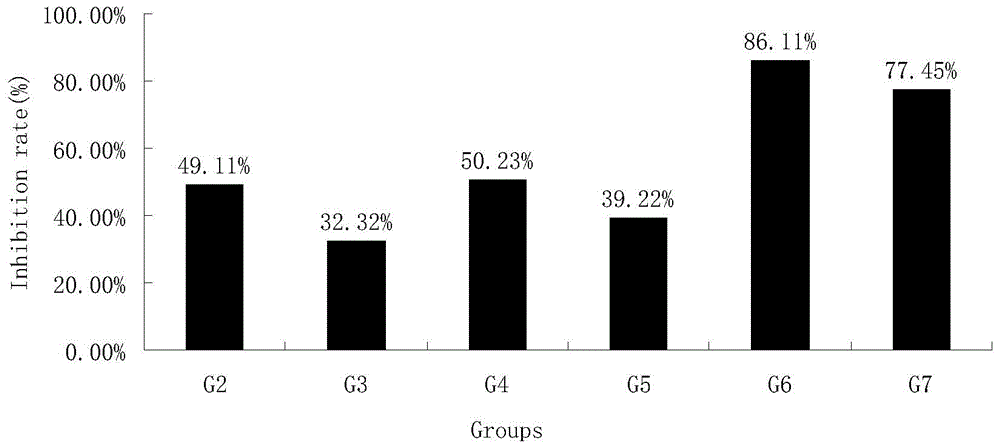
[0067] Tumor weight (g) and tumor inhibition rate (%) when table 4 was treated on the 21st day
[0068]
[0069]
[0070] The data analysis software was SPSS, and the average tumor weight of each group was expressed as mean±SD. According to the T test, the tumor inhibition rate was calculated = (tumor weight of the negative group ...
Embodiment 3
[0074] Embodiment 3 HM-3 combines docetaxel, oxaliplatin to the inhibitory effect of human breast cancer MDA-MB-231 nude mouse xenograft tumor
[0075] Breast cancer tissues in vigorous growth stage were taken and ground under sterile conditions, and prepared into 1×10 7 / mL cell suspension, planted in the right armpit of mice with 0.1 mL. Other model preparation methods are referring to embodiment 1.
[0076] The test was divided into 8 groups, including 12 rats in the negative control group, 8 rats in the HM-3 group, and 10 rats in each of the other treatment groups. See Table 1 in Example 1 for the specific dosage regimen. After the 21st day of treatment, the nude mice were dissected, tumors were taken, and the tumor inhibition rate was determined.
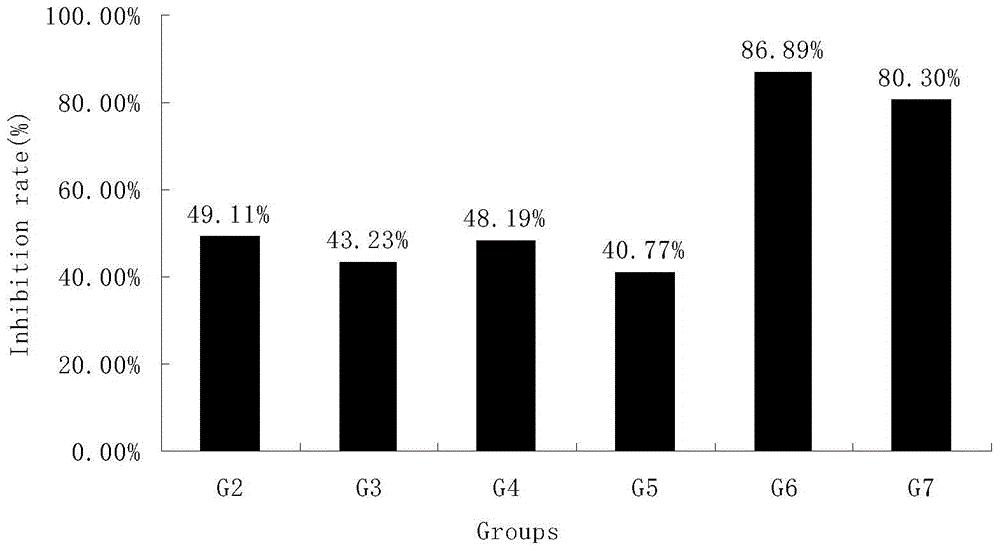
[0077] Table 5 Tumor weight (g) and tumor inhibition rate (%)
[0078]
[0079] The data analysis software was SPSS, and the average tumor weight of each group was expressed as mean±SD. Tumor inhibition rate=(tumor weig...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com