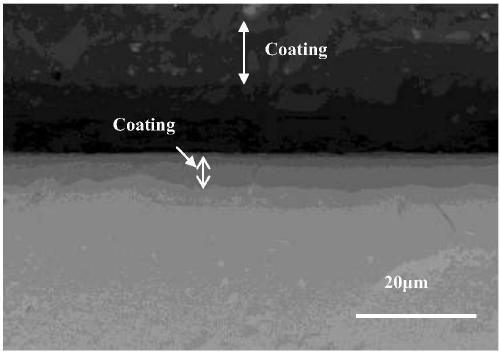
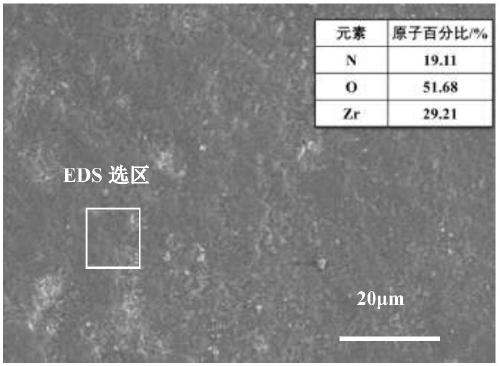
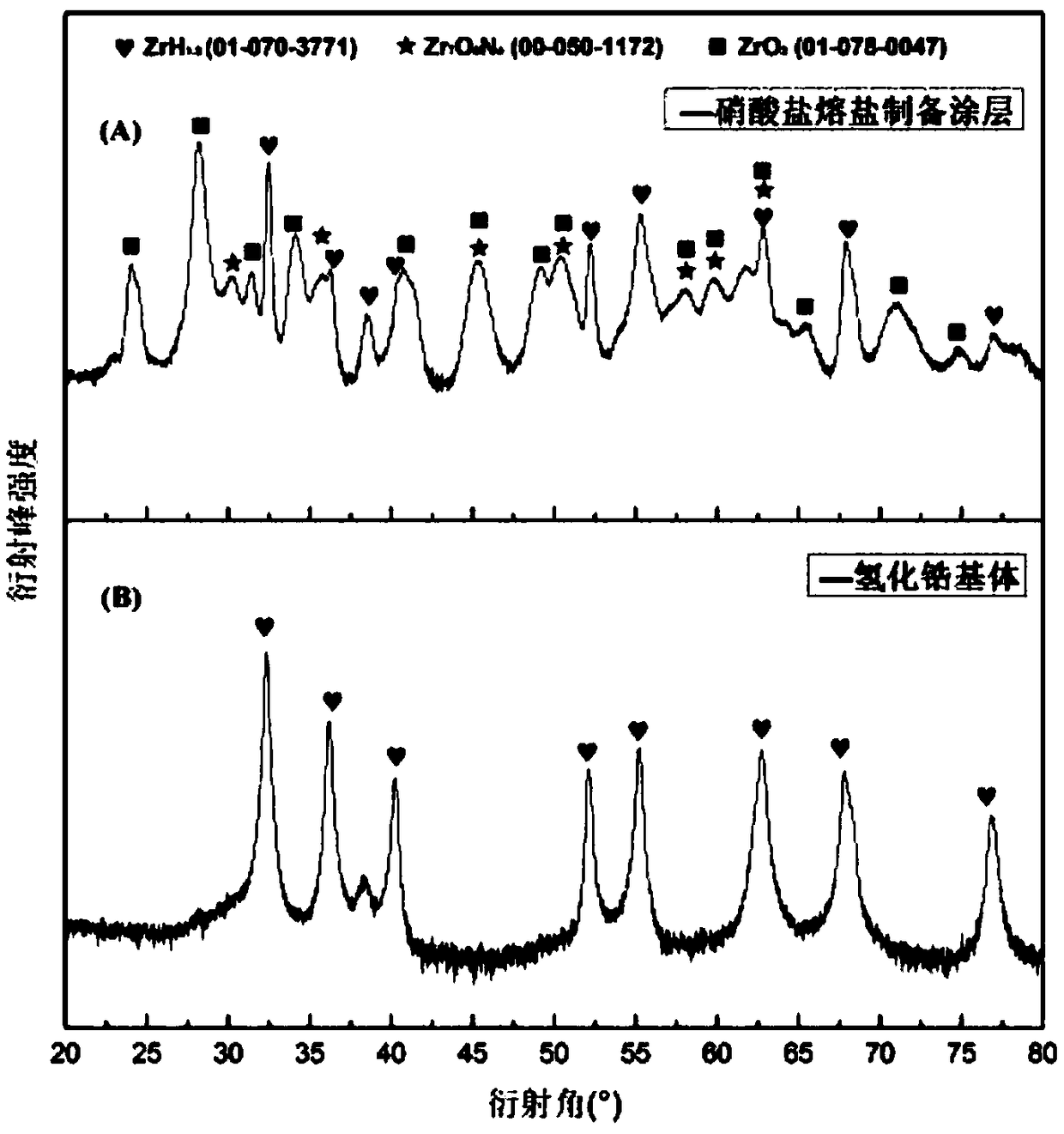
A preparation method of a hydrogen permeation-resistant coating on a metal hydride surface in a molten salt system
A hydride and metal technology, applied in the direction of metal material coating process, coating, solid diffusion coating, etc., can solve the problem that the inner wall of the deep hole cannot be protected by a corrosion-resistant layer, and achieves hard peeling and cracking. The effect of stable hydrogen permeation performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0027] 1. Hydride workpiece pretreatment:
[0028] The surface of Φ20mm×20mm zirconium hydride is degreased, cleaned and polished, and the zirconium hydride H / Zr=1.0.
[0029] 2. Compound nitrate preparation:
[0030] According to the mass percentage, it is prepared with 30% of sodium nitrite, 10% of sodium nitrate and 60% of potassium nitrate, heated to 280°C to fully melt each component, stir evenly, cool and solidify, then crush to obtain compound nitrate.
[0031] 3. Molten salt heat treatment of zirconium hydride:
[0032] (1) Put the composite nitrate and the zirconium hydride workpiece together into a stainless steel crucible and place it in a heating furnace.
[0033] (2) The heating furnace is raised from room temperature to 150°C at a rate of 10°C / hour, kept for 1 hour, continued to heat up to 450°C, kept at a temperature of 20 hours, cooled to 150°C at 600°C / hour, and the hydride workpiece was taken out and cooled to room temperature.
[0034] 4. Post-processing ...
Embodiment 2
[0038] 1. Hydride workpiece pretreatment:
[0039] Degrease, clean and polish the surface of Φ20mm×20mm zirconium hydride, zirconium hydride H / Zr=1.6.
[0040] 2. Compound nitrate preparation:
[0041] According to mass percentage, sodium nitrate: 60% and potassium nitrate: 40% are prepared, melted at 300°C, cooled and solidified, and broken to obtain compound nitrate.
[0042] 3. Molten salt heat treatment of zirconium hydride:
[0043] (1) Put the composite nitrate and the zirconium hydride workpiece together into a stainless steel crucible and place it in a heating furnace.
[0044] (2) The heating furnace is raised from room temperature at 600°C / hour to 200°C, kept for 1 hour, continued to heat up to 650°C, kept at temperature for 10 hours, cooled to 280°C at 600°C / hour, and the hydride workpiece was taken out and cooled to room temperature.
[0045] 4. Post-processing of hydride workpieces:
[0046] The zirconium hydride workpiece was placed in deionized water, ultrason...
Embodiment 3
[0048] 1. Hydride workpiece pretreatment:
[0049] The Φ20mm×20mm titanium hydride surface is degreased, cleaned and polished, and the zirconium hydride H / Ti=1.0.
[0050] 2. Compound nitrate preparation:
[0051] According to mass percentage, it is prepared as sodium nitrite: 50%, sodium nitrate: 30%, potassium nitrate: 20%, is heated to 180°C to fully melt each component, stirs evenly, cools and solidifies, and breaks to obtain compound nitrate.
[0052] 3. Molten salt heat treatment of titanium hydride:
[0053] (1) Put the composite nitrate and the titanium hydride workpiece together into a stainless steel crucible and place it in a heating furnace.
[0054] (2) The heating furnace rises from room temperature at 600°C / hour to 200°C, holds for 10 hours, continues to heat up to 650°C, holds for 40 hours, cools to 280°C at 600°C / hour, takes out the workpiece and cools to room temperature.
[0055] 4. Workpiece post-processing:
[0056] The titanium hydride workpiece was p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com