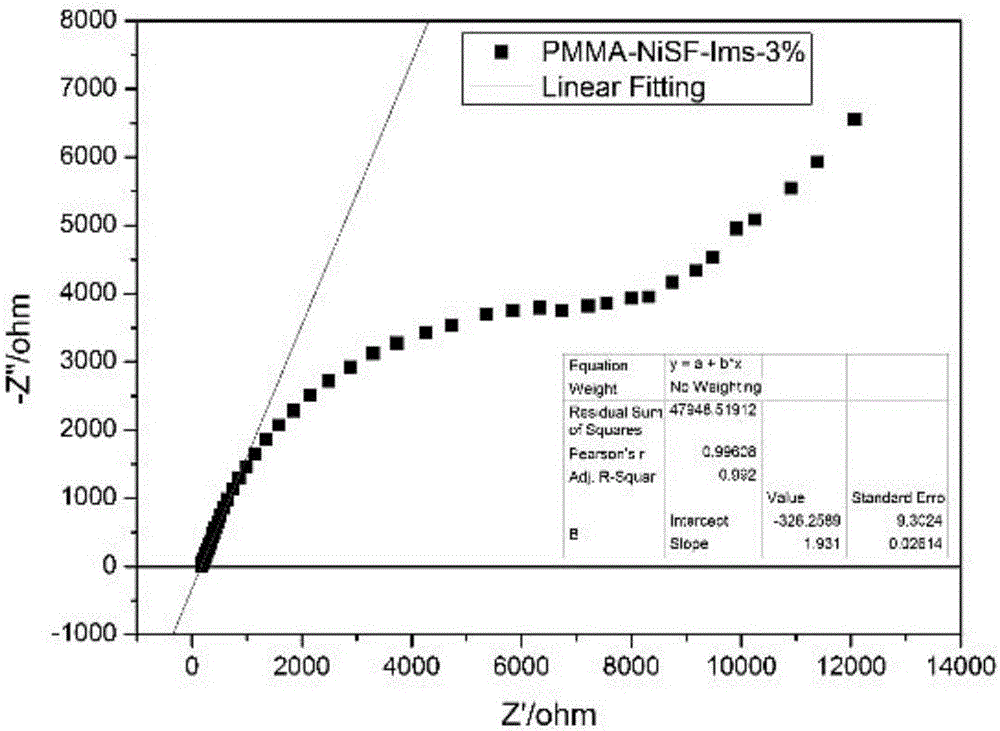
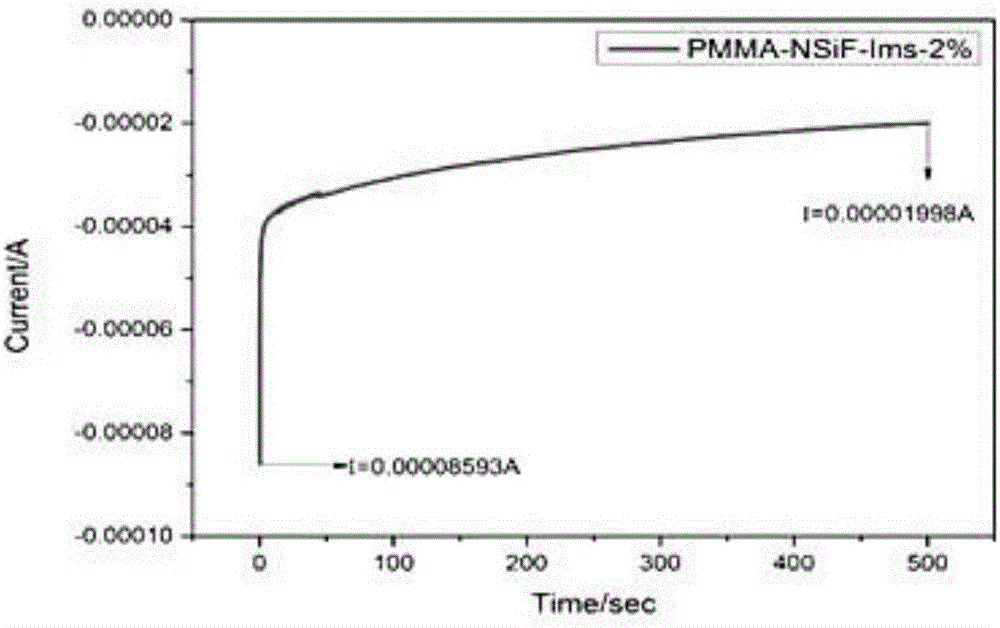
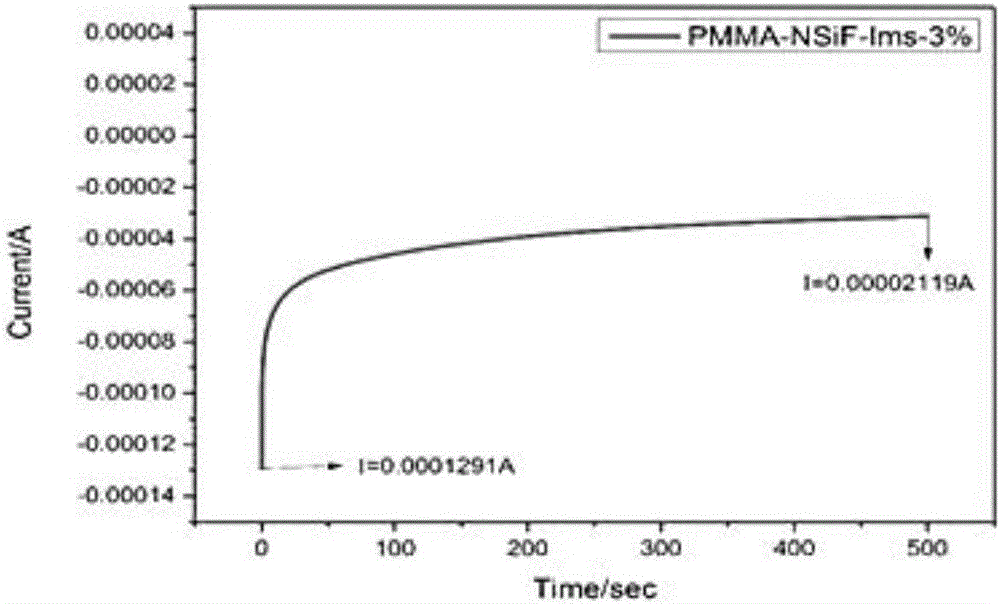
Preparation method of all-solid-state polymer electrolyte
An all-solid polymer and electrolyte technology, used in circuits, electrical components, secondary batteries, etc., can solve problems such as explosions, potential safety hazards, and easily induced combustion.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0022] The invention provides a method for preparing an all-solid polymer electrolyte, comprising the following steps: 2 In the atmosphere, mix the ammonia-free modifier and methylimidazole, heat in an oil bath, and obtain a quaternary ammonium salt after reaction; graft PEG sulfonic acid groups on the quaternary ammonium salt to obtain PEG sulfonate, and imidazolium salt Dissolve in THF, then add described PEG sulfonate, obtain reaction product A after the reaction; Described reaction product A and SiO 2 The aqueous solution is mixed, and after the reaction, a methylimidazole-containing fluid is obtained; Methyl methacrylate (MMA), LiClO 4 -PC solution, azobisisobutyronitrile (AIBN) and the fluid containing methylimidazole are mixed, stirred, and reacted to obtain a prepolymer; the prepolymer is coated on the surface of aluminum foil and heated to obtain an all-solid state polymer electrolyte.
[0023] As a preferred solution, the present invention has no special restrictio...
Embodiment 1
[0035] Accurately weigh 3.0000g of ammonia-free modifier and 3.7500g of methylimidazole, and pour them into a three-necked flask. The whole process is carried out under N 2 Carried out in the atmosphere, the viscous quaternary ammonium salt is obtained after the reaction, and the reaction conditions are 80°C, 72h;
[0036] Graft the PEG sulfonic acid group to the quaternary ammonium salt, accurately weigh 3.6553g of imidazolium salt and dissolve it in THF, then weigh 15.25g of PEG sulfonate and add it, and the reaction lasts at room temperature for 1h. After the reaction is completed, filter the product , wash with THF, obtain reaction product A;
[0037] First weigh 3.15g of reaction product A and dissolve it in 15ml of absolute ethanol, then weigh 1.17g of nano-SiO 2 Dissolve in about 28ml distilled water; SiO 2 The aqueous solution was added to the ethanol solution of the reaction product A, and the reaction was carried out in the flask, and stirred magnetically for 24 ho...
Embodiment 2
[0041] Accurately weigh 3.0000g of ammonia-free modifier and 3.7500g of methylimidazole into a three-necked flask, and the whole process is carried out under N 2 Carried out in the atmosphere, the viscous quaternary ammonium salt is obtained after the reaction, and the reaction conditions are 80°C, 72h;
[0042] Graft the PEG sulfonic acid group to the quaternary ammonium salt, accurately weigh 3.6553g of imidazolium salt and dissolve it in THF, then weigh 15.25g of PEG sulfonate and add it, and the reaction lasts at room temperature for 1h. After the reaction is completed, filter the product , wash with THF, obtain reaction product A;
[0043] First weigh 3.15g of reaction product A and dissolve it in 15ml of absolute ethanol, then weigh 1.17g of nano-SiO 2 Dissolve in about 28ml distilled water; SiO 2 The aqueous solution was added to the ethanol solution of the reaction product A, and the reaction was carried out in the flask, and stirred magnetically for 24 hours at room...
PUM
| Property | Measurement | Unit |
|---|---|---|
| electrical conductivity | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com