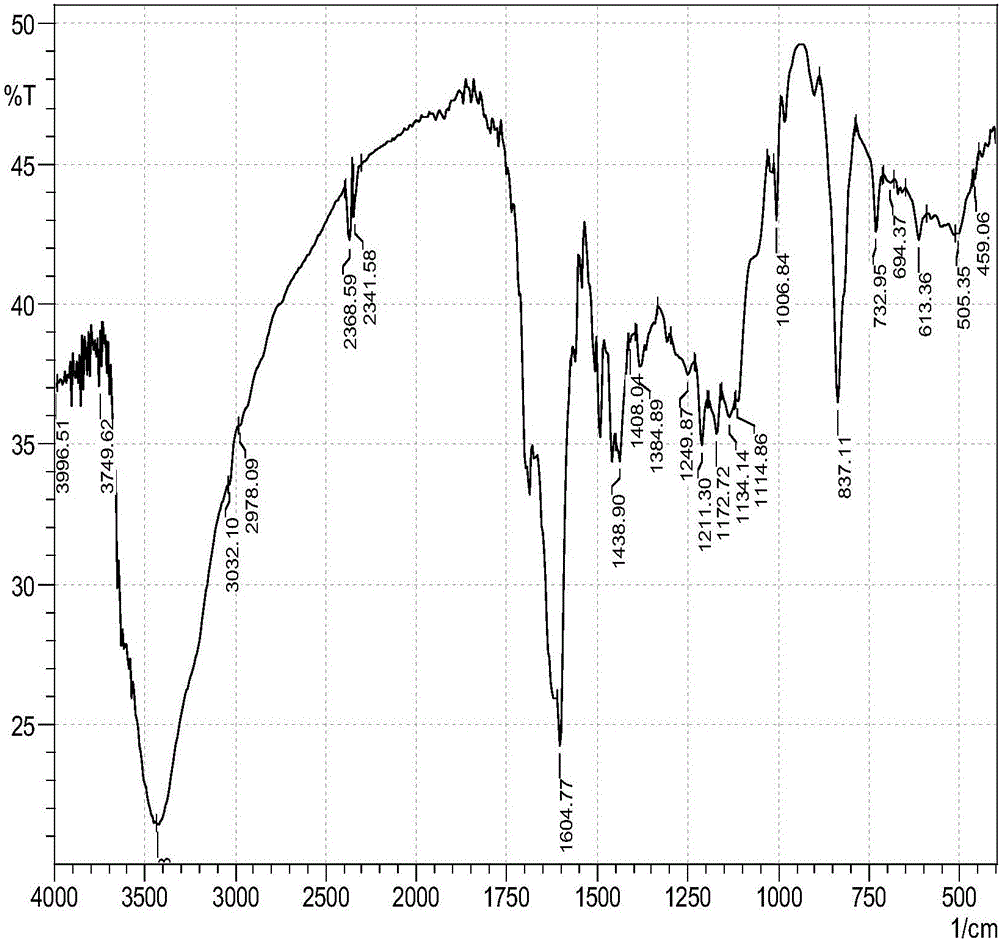
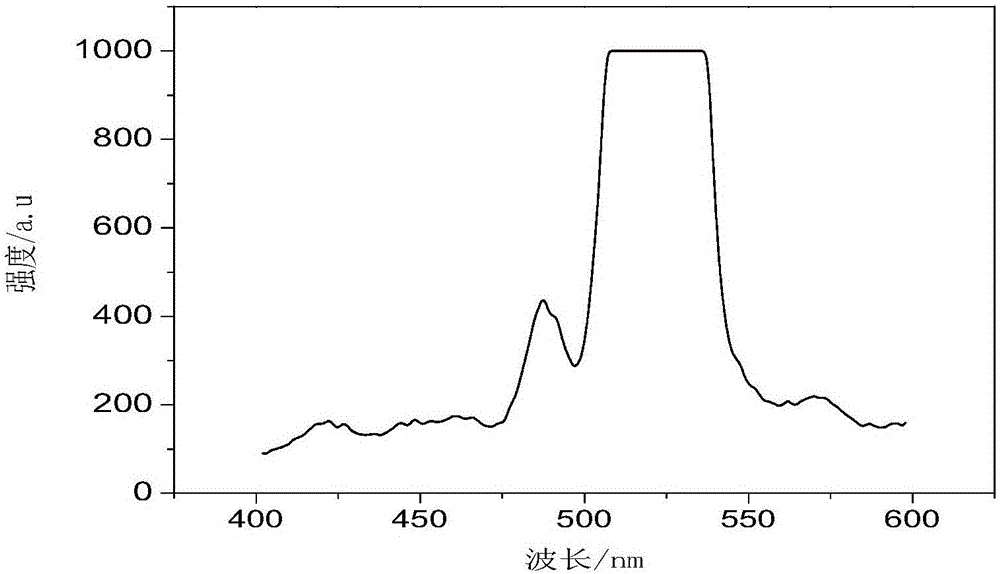
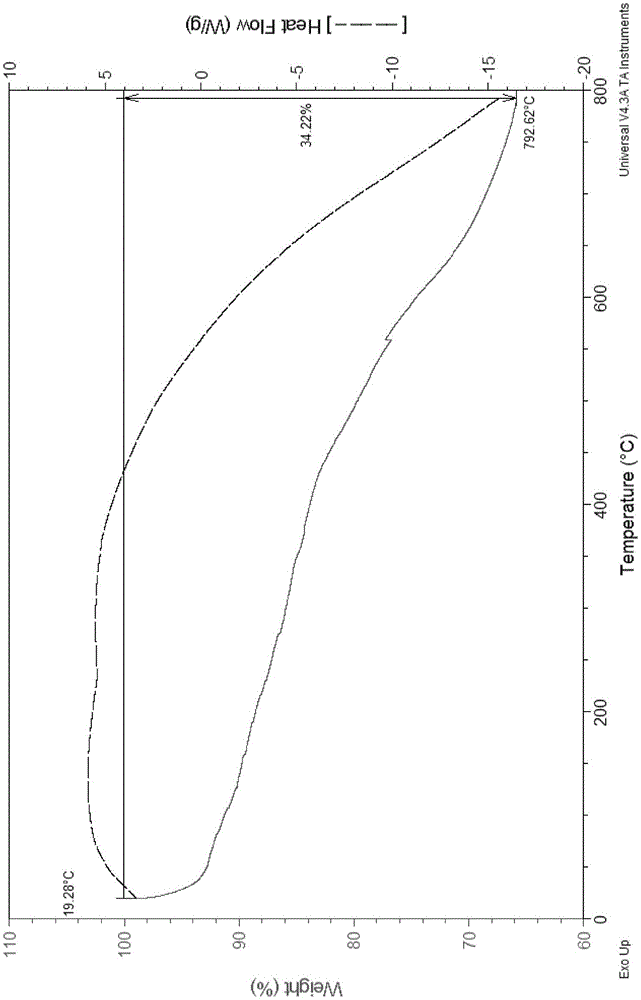
Porous pyrenyl organic framework material rich in hydroxyl and preparation method thereof
An organic framework, hydroxyl-rich technology, applied in luminescent materials, chemical instruments and methods, etc., can solve the problems of difficult synthesis, unstable structure, and environmental sensitivity of MOFs materials.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0038] The invention provides a method for preparing a hydroxyl-rich porous pyrene-based organic framework material, comprising the following steps:
[0039] Under an inert atmosphere, the phenolic compound and the tetraaldehyde group aromatic aldehyde are subjected to polycondensation reaction in an organic solvent to obtain a hydroxyl-rich porous pyrene-based organic framework material;
[0040] Described phenolic compound is polyhydric phenol or polyhydric naphthol;
[0041] The tetraaldehyde-based aromatic aldehyde contains 4 to 8 benzene rings, and the four aldehyde groups are respectively connected to four different benzene rings;
[0042] The organic solvent is an alcohol organic solvent or an ether organic solvent.
[0043] In the present invention, the phenolic compound is polyhydric phenol or polyhydric naphthol, preferably trihydric phenol or dihydric naphthol. In the present invention, the phenolic compound preferably has the structure shown in formula I or formu...
Embodiment 1
[0057] Put pyrene (1.0g, 4.9mmol) in a clean beaker, pour nitrobenzene (20mL) into the beaker and stir to dissolve, after it is completely dissolved, slowly add liquid bromine (1.12mL, 21.8mmol) dropwise , The dripping speed is controlled within 1h. The temperature of the reaction system was raised to 120°C, and the reaction time was set for 14 hours. The color of the reaction solution turned to khaki. The crude product obtained by filtration was ultrasonically cleaned with ethanol three times to obtain a light green solid, 1,3,6,8-tetrabromopyrene.
[0058] In the round bottom flask of 250mL, add palladium chloride (0.898g, 0.005mol), triphenylphosphine (0.6602g, 2.5mmol) and toluene (6ml) solution, under the protection of nitrogen, reaction solution is heated to solid matter All dissolved, the whole reaction solution is yellow and transparent, the reaction temperature is controlled at 130°C, the oil bath is turned off when the time is up, and after adding 1mL hydrazine hydra...
Embodiment 2
[0069] Put pyrene (1.0g, 4.9mmol) in a clean beaker, pour nitrobenzene (20mL) into the beaker and stir to dissolve, after it is completely dissolved, slowly add liquid bromine (1.12mL, 21.8mmol) dropwise , The dripping speed is controlled within 1h. The temperature of the reaction system was raised to 120°C, and the reaction time was set for 14 hours. The color of the reaction solution turned to khaki. The crude product obtained by filtration was ultrasonically cleaned with ethanol three times to obtain a light green solid, 1,3,6,8-tetrabromopyrene.
[0070] In the round bottom flask of 250mL, add palladium chloride (0.898g, 0.005mol), triphenylphosphine (0.6602g, 2.5mmol) and dimethyl sulfoxide (6ml) solution, under the protection of nitrogen, the reaction solution is heated When the solid matter is completely dissolved, the entire reaction solution is yellow and transparent. The reaction temperature is controlled at 140°C. When the time is up, the oil bath is turned off. Aft...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Aperture | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com