Organic composite high-temperature proton exchange membrane and preparation method thereof
A proton exchange membrane and composite technology, applied in the field of organic composite high-temperature proton exchange membrane and its preparation, can solve the problems of complex water and heat management of batteries, PEM dehydration, and affecting battery performance stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0059] Preparation of Composite High Temperature Proton Exchange Membrane
[0060] In a preferred embodiment of the present invention, the preparation method of the composite high-temperature proton exchange membrane is as follows:
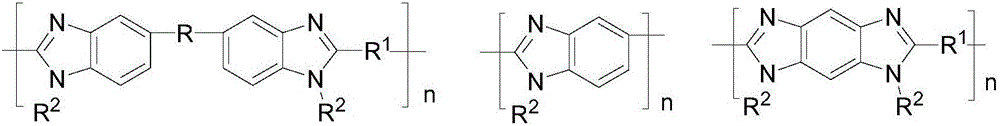
[0061] Provide polybenzimidazole type compound A and strong basic polymer B;
[0062] Under the protection of an inert gas, dissolve the mixture of the two in an organic solvent to form a mixed solution with a certain solid content;
[0063] Remove the insolubles by filtration to obtain a mixed filtrate;
[0064] Degassing the mixed filtrate;
[0065] The degassed mixed filtrate is formed into a membrane to obtain a composite high-temperature proton exchange membrane.
[0066] In a preferred embodiment of the present invention, the molar ratio of the polybenzimidazole type compound A to the strongly basic polymer B is 1:0.1-20.
[0067] In a preferred embodiment of the present invention, the organic solvent is a strong polar organic solvent, m...
Embodiment 1
[0079] Preparation of m-PBI and 3,5-polypyridine (molar ratio 1:0.1) composite membrane:
[0080]
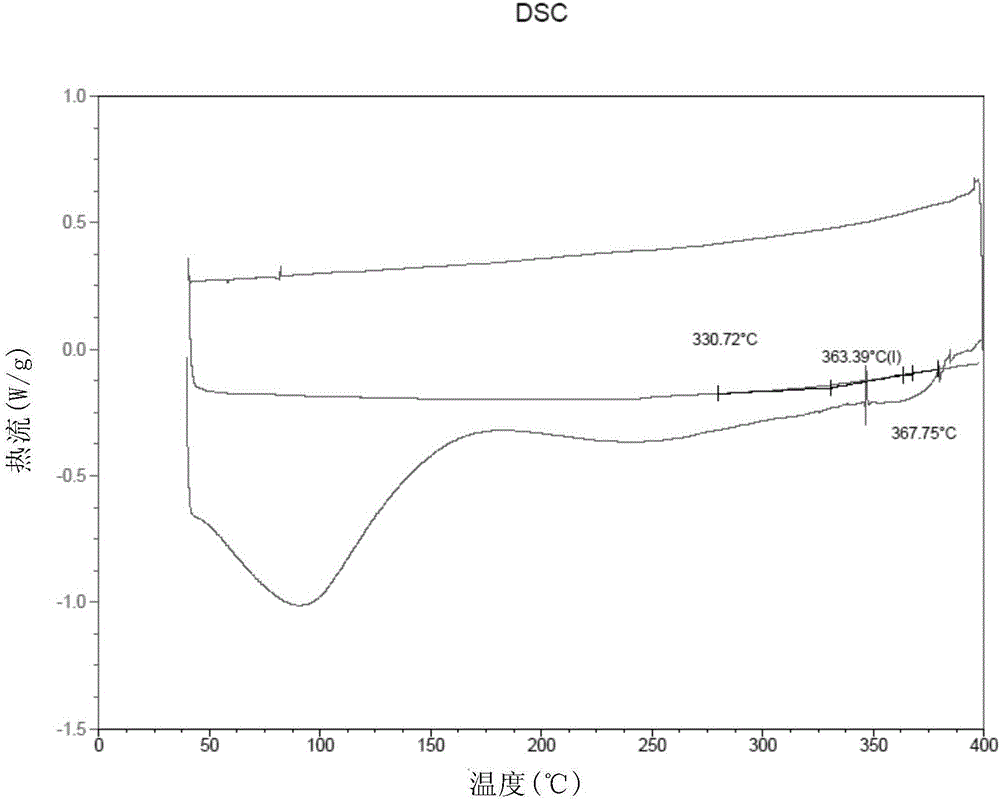
[0081] According to the molar ratio of 1:0.1, dry m-PBI (616.7mg, 2mmol) and 3,5-polypyridine (15.4mg, 0.2mmol) were weighed. Under the protection of nitrogen, the mixture of the two was dissolved in dry DMSO (dimethyl sulfoxide, 12.010g) to form a solution with a solid content of 5%, filtered to remove insoluble matter, and the filtrate was degassed After treatment, it was cast onto a glass plate of 10×10 cm, and then placed in a blast oven to dry at 80°C for two hours, and then further heated to 120°C for one hour to obtain a composite high-temperature proton exchange membrane. The thickness of this film is 24 μ m (micrometer), DSC test (results such as figure 1 shown in ) shows its glass transition temperature T g =365°C, the mechanical property test shows that its tensile strength is 103MPa.
[0082] It is known that the tensile strength of the film prepared by m-PBI i...
Embodiment 2
[0085] Preparation of p-PBI and 2,6-polypyridine (molar ratio 1:0.2) composite membrane:
[0086]
[0087] According to the molar ratio of 1:0.2, dry p-PBI (616.7mg, 2mmol) and 2,6-polypyridine (30.8mg, 0.4mmol) were weighed. Under the protection of nitrogen, the mixture of the two was dissolved in dry DMAC (N,N-dimethylacetamide, 10.144g) to form a solution with a solid content of 6%, filtered, and the insoluble matter was filtered off. After degassing, the filtrate was cast onto a 10×10cm glass plate, and then dried in a blast oven at 80°C for two hours, and then further heated to 120°C for one hour to obtain a composite high-temperature proton exchange membrane.
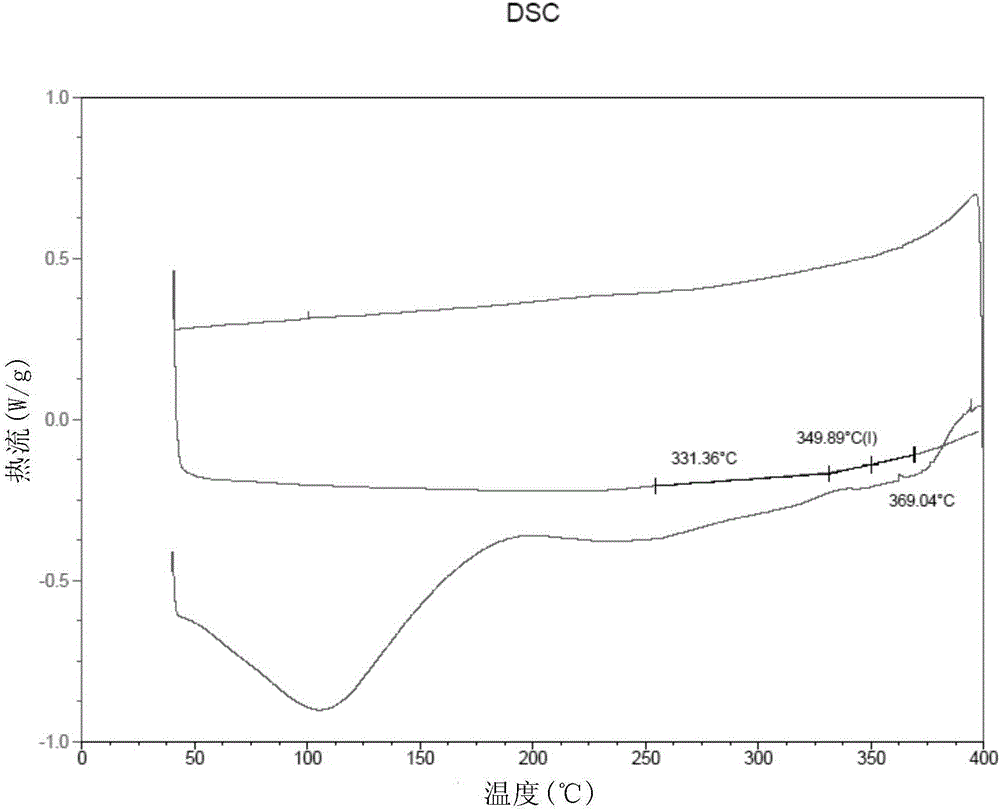
[0088] The thickness of this film is 25 μ m (micrometer), DSC test (results such as figure 2 shown in ) shows its glass transition temperature T g =360°C, the mechanical property test shows that its tensile strength is 101MPa.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Thickness | aaaaa | aaaaa |
| Glass transition temperature | aaaaa | aaaaa |
| Tensile strength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com