Synthesizing method of trometamol prostaglandin F2alpha
A technology of trometamol and prostaglandins, which is applied in the preparation of amino hydroxyl compounds, organic chemical methods, chemical instruments and methods, etc., can solve the problems of unsuitability for industrial production, difficult control of reaction time, high price, etc., and achieve selectivity Outstanding, low-cost, and easy-to-purchase results
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0029] The present invention will be further described below in conjunction with the accompanying drawings and embodiments.
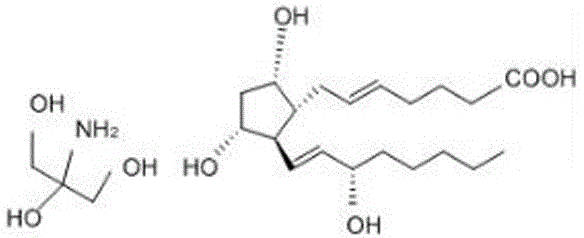
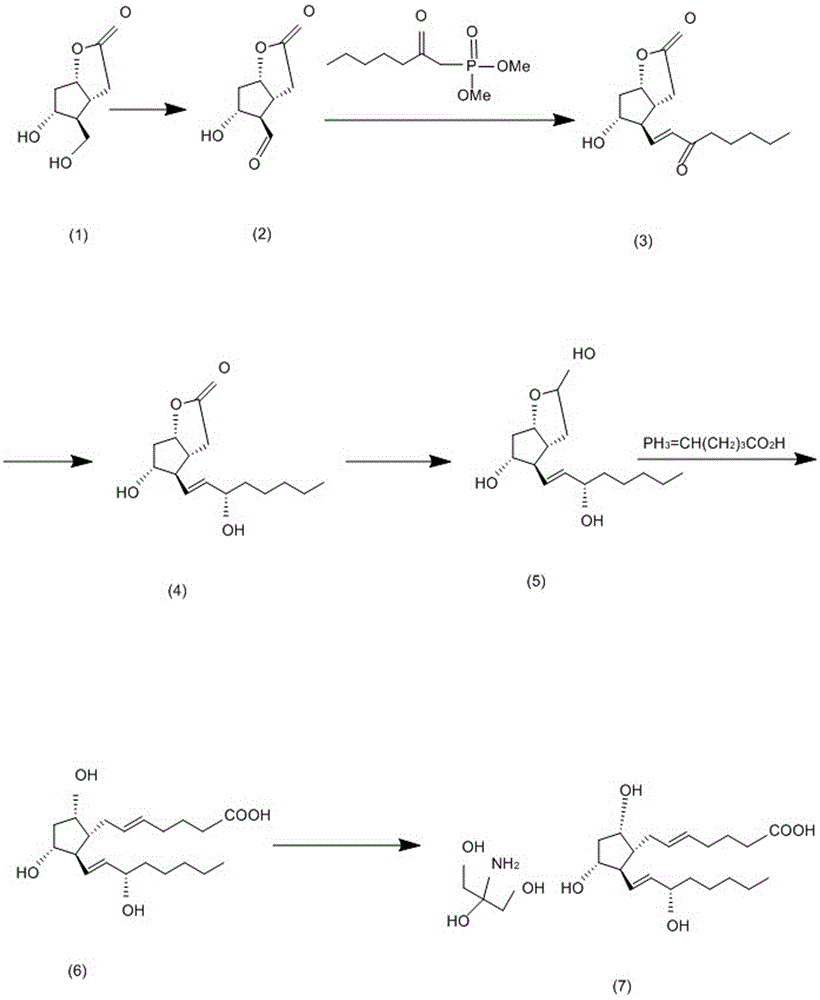
[0030] Such as figure 1 , 2 Shown, the synthetic method of trometamol prostaglandin F2α provided by the present invention, take compound (-)-Corey lactone diol as raw material, make structural formula after oxidation reaction: The lactone aldehyde, the lactone aldehyde and the structural formula are The lower side chain of the Wittig-Honner reaction is spliced to obtain the structural formula as The alkene, the dicarbonyl in the alkene is reduced to obtain the structural formula: Alcohols, and then with the structural formula as Prostaglandin F2α is obtained through the Ylide-Wittig reaction on the upper side chain of the product, and then the prostaglandin F2α is dissolved in tromethamine and crystallized to obtain tromethamine prostaglandin F2α.
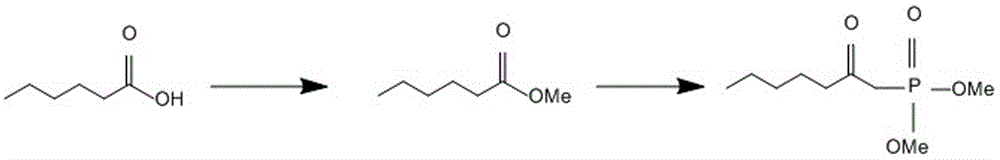
[0031] Such as image 3 shown, where the structural formula is The lower side chain of is p...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com