A kind of efficient preparation method of bazedoxifene
A bazedoxifene, high-efficiency technology, applied in the field of efficient preparation of bazedoxifene, can solve the problems of hydrogen ignition, high difficulty in preparation of bazedoxifene, high price, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
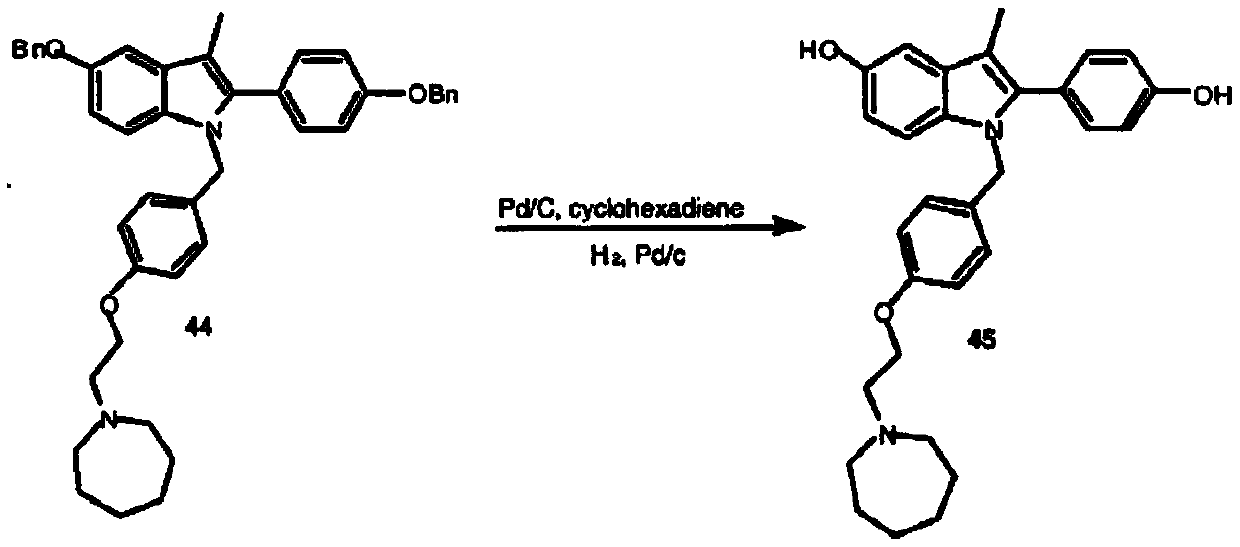
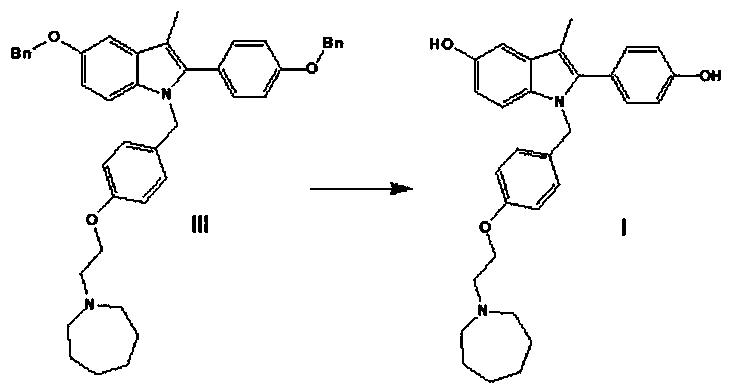
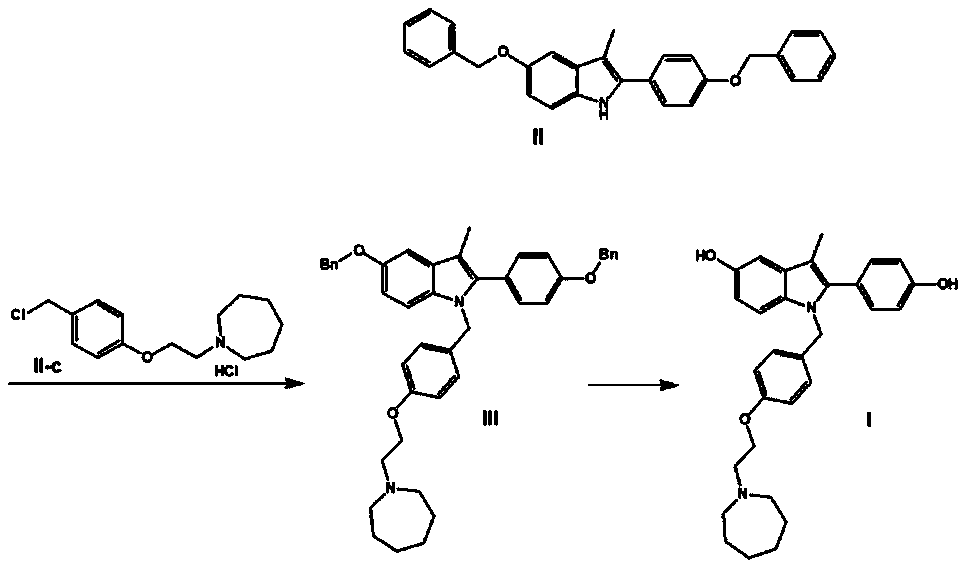
[0079] The synthetic route of this experimental example is as follows:
[0080]
[0081] In the formula, Bn refers to benzyl (C 6 h 6 CH 2 -).
[0082] 1. Preparation of 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole
[0083]Weigh 10g of 4'-benzyloxy-2-bromopropiophenone (II-a), 15g of p-benzyloxyaniline hydrochloride (II-b), 50mL of N,N-dimethylformamide and 8 mL of triethylamine was reacted at 115°C for 4 hours. Thin-layer chromatography (TLC) detected that the reaction was complete, and the reaction solution was poured into 250 mL of ice water to precipitate solids, filtered by suction, and the obtained crude product was stirred and beaten with 20 mL of methanol, and vacuum-dried at 40 ° C for 24 hours to obtain 12.5 g of a yellow-brown solid product, namely It is 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole (intermediate II), and the yield is 94.6%.
[0084] MS [M+H]: 420.52.
[0085] 1 HNMR (DMSO-d 6 ): 2.30ppm(s,3H), 5.21ppm(m,4H), 6.73ppm(d...
Embodiment 2
[0095] 1. Preparation of 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole
[0096] Weigh 10g of 4'-benzyloxy-2-bromopropiophenone (II-a), 15g of p-benzyloxyaniline hydrochloride (II-b), 50mL of N,N-dimethylformamide and 8 mL of triethylamine was reacted at 118°C for 3 hours. Thin-layer chromatography (TLC) detected that the reaction was complete. Pour the reaction solution into 250 mL of ice water to precipitate a solid, and filter it with suction. The obtained crude product was stirred and beaten with 20 mL of methanol, and the wet product was vacuum-dried at 42°C for 24 hours to obtain 12.5 g of a yellow-brown solid product. , namely 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole (intermediate II), with a yield of 94.6%.
[0097] 2. Preparation of 1-(4-(2-(azepan-1-yl)ethoxy)benzyl)-5-(benzyloxy)-2-(4-(benzyloxy)phenyl) -3-Methyl-1H-indole
[0098] Weigh 3g of 60% NaH, 8g of 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole (intermediate II), 7g of 1-[ ...
Embodiment 3
[0102] 1. Preparation of 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole
[0103] Weigh 10g of 4'-benzyloxy-2-bromopropiophenone (II-a), 15g of p-benzyloxyaniline hydrochloride (II-b), 50mL of N,N-dimethylformamide and 8 mL of triethylamine was reacted at 115°C for 4 hours. Thin-layer chromatography (TLC) detected that the reaction was complete. Pour the reaction solution into 250 mL of ice water to precipitate a solid, and filter it with suction. The obtained crude product was stirred and beaten with 20 mL of methanol, and the wet product was vacuum-dried at 40°C for 24 hours to obtain 12.5 g of a yellow-brown solid product. , namely 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole (intermediate II), with a yield of 94.6%.
[0104] 2. Preparation of 1-(4-(2-(azepan-1-yl)ethoxy)benzyl)-5-(benzyloxy)-2-(4-(benzyloxy)phenyl) -3-Methyl-1H-indole
[0105] Weigh 3g of 60% NaH, 8g of 5-benzyloxy-2-[(4-benzyloxy)phenyl]-3-methyl-1H-indole (intermediate II), 7g of 1-[ ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com