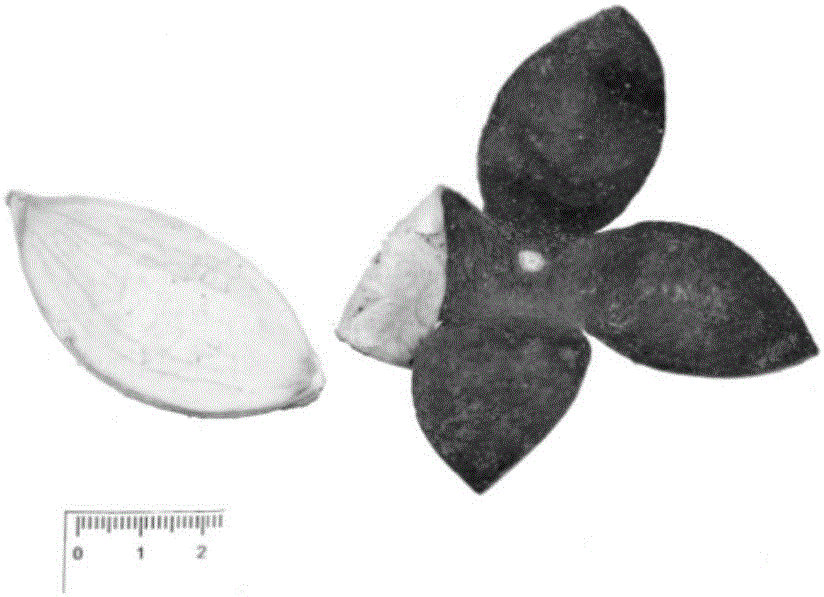
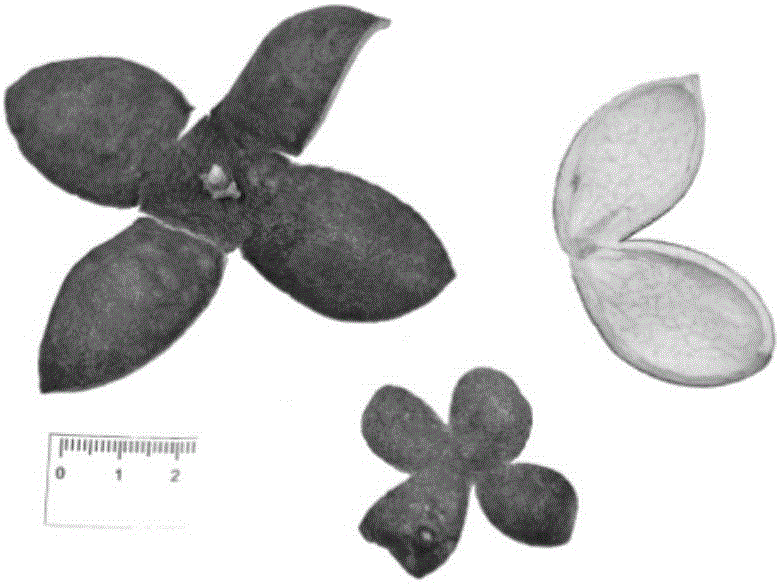
Pericarpium citri reticulatae viride reference extract preparation method
A technology of extract and green bark, which is applied in the field of preparation of green bark control extract, to achieve uniform use, ensure consistency, and stable properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0112] Embodiment 1: the preparation of Qingpi reference extract
[0113] The present embodiment provides the preparation method of Qingpi reference extract of the present invention, and its method flow chart is as follows Figure 21 shown.
[0114] 1: Screening of raw materials and medicinal materials
[0115] In this step, the combination of thin layer chromatography and high performance liquid chromatography is used to screen the raw medicinal materials.
[0116] 1 Thin-layer chromatography screening of raw materials
[0117] 1.1 Preparation of the test product
[0118] Preparation of chemical reference solution: take hesperidin, nobietin, and tangeretin reference substances, accurately weigh them, and prepare 0.1 mg / ml solutions with methanol respectively.
[0119] The preparation of medicinal material need testing solution: get green peel A, green peel B, green peel C, green peel D, green peel E, green peel F, green peel G, green peel H, green peel I, green peel J med...
Embodiment 2
[0242] The character analysis of embodiment 2 Qingpi contrast extract
[0243] 1. Appearance: The Qingpi control extracts QPERS-1 and QPERS-2 obtained in Example 1 are both yellow-green dry powders.
[0244] 2. Moisture determination: Carry out according to Appendix IX G of the Chinese Pharmacopoeia of the 2010 edition (reduced pressure drying method). The test result shows that the water content of QPERS1 is 2.70%, and the water content of QPERS2 is 2.30%.
[0245] 3. Consistency test: prepare 10 batches of Sihua Qingpi ERS (QPERS-1) (6 batches) and a Qingpi ERS (QPERS-2) (4 batches) according to the method of Example 1, and each batch is measured The TLC detection results for flavonoid glycosides and flavonoid aglycone components showed little difference, and the fluorescent spots of hesperidin, tangeretin and nobiletin were all clearly displayed, and the distribution was very consistent; The content of hesperidin was detected by liquid chromatography within the standard r...
Embodiment 3
[0253] This example provides the application of the reference extract of Pseudomonas chinensis in Example 1.
[0254] Commercial medicinal materials were analyzed by thin-layer chromatography and high-performance liquid chromatography, respectively, with chemical reference substance and Qingpi reference extract as reference substances.
[0255] 1 TLC
[0256] 1.1 Sample preparation
[0257] Chemical reference solution: Accurately weigh 2.5mg each of hesperidin, nobiletin, tangeretin, neohesperidin, and naringin, put them in a 25ml volumetric flask, add 20ml of methanol, and process with ultrasonic (500w) for 15 minutes to Dissolve completely, let cool, make up to 25ml mark and pass through 0.22μm filter membrane.
[0258] Qingpi reference extract solution: Accurately weigh 20 mg each of Qingpi reference extracts QPERS-1 and QPERS-2 prepared in Example 1, put them in a 2ml measuring bottle, add 1.5ml of methanol respectively, ultrasonically (500w) for 15 minutes, put Cool, a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| pore size | aaaaa | aaaaa |
| wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com