Midodrine hydrochloride medicinal composition
A technology of midodrine hydrochloride and a composition, which is applied in directions such as drug combination, pill delivery, blood diseases, etc., can solve the problems of difficult mixing, complicated process, poor stability, etc., and achieves simplified production process, simple production process, and improved stability. sexual effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
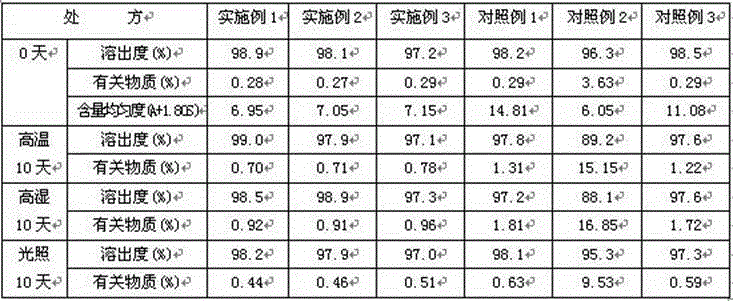
[0023] Example 1 Preparation of Midodrine Hydrochloride Tablets
[0024] Recipe:
[0025]
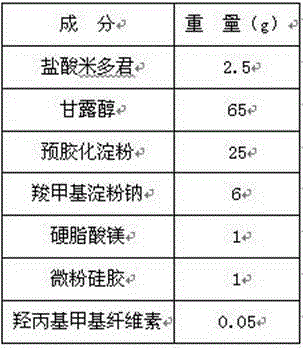
[0026] Weigh midodrine hydrochloride that has passed through a 100-mesh sieve, mannitol, pregelatinized starch, sodium carboxymethyl starch, magnesium stearate, and micronized silica gel that have passed through a 60-mesh sieve according to the weight parts of the components; Midodrine and part of the mannitol are mixed uniformly in equal increments, then pregelatinized starch, sodium carboxymethyl starch, and the remaining mannitol are added to mix evenly, and the hydroxypropyl methylcellulose aqueous solution is added and mixed evenly to make a soft material 、Sieving granulation; drying at 55℃-65℃ to dryness, the moisture content is controlled within 1%-5.0%; sieve the dried granules, add magnesium stearate and micronized silica gel, mix well, and mix well The granules are compressed.
Embodiment 3
[0032] Recipe:
[0033]
[0034] The preparation method is the same as in Example 1.
[0035] Control sample 1 Preparation of midodrine hydrochloride tablets (refer to Japanese patent)
[0036] Recipe:
[0037]
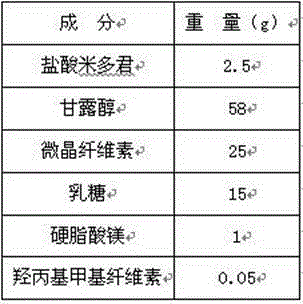
[0038] Weigh out midodrine hydrochloride that has passed through a 100-mesh sieve, and mannitol, microcrystalline cellulose, lactose, sodium carboxymethyl starch, and magnesium stearate that have passed through a 60-mesh sieve according to the weight of the components; Methyl cellulose is dissolved in the aqueous solution for later use; the midodrine hydrochloride and mannitol are mixed uniformly in equal increments, the soft material is made from the hydroxypropyl methyl cellulose aqueous solution, and then granulated by sieving; microcrystalline cellulose, Mix lactose evenly, add hydroxypropyl methylcellulose aqueous solution to make soft material, sieving and granulation; dry the two kinds of granules at 55℃-65℃ until the moisture content is within 1.0%-5.0%; sieving the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com