Anti-hyperlipidemia protein vaccine aiming at PCSK9 (Proprotein convertase subtilisin/kexin type 9)
An anti-hyperlipidemic and protein vaccine technology, applied in the field of biomedicine, can solve the problems of high cost, short action time, complicated purification process, etc., and achieve the effects of avoiding drug tolerance and high cost, long action time, and good application prospect.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0036] In addition, the present invention also provides a preparation method of the above-mentioned protein vaccine. The method comprises the steps of:
[0037] 1. Construction of vectors of PCSK9 genes with different structural domains
[0038] The cDNA fragments of the catalytic domain and the C-terminal of PCSK9 were amplified by PCR reaction using the cDNA sequence, and constructed into the expression vector (pET32a) by endonuclease digestion and DNA ligase ligation, respectively denoted as pET32a-pcsk9-I and pET32a -pcsk9-II.
[0039] 2. Expression and purification of PCSK9-Ⅰ and PCSK9-Ⅱ proteins with different domains
[0040] The recombinant expression plasmid was transformed into Escherichia coli strain BL21(DE3) for expression, and the strains with high expression of PCSK9-Ⅰ and PCSK9-Ⅱ proteins were screened out respectively. The recombinant protein mainly existed in the form of inclusion body, and the inclusion body protein was treated with different concentration...
Embodiment 1
[0045] Example 1 Obtaining of different structural domain genes of PCSK9 and construction of vectors
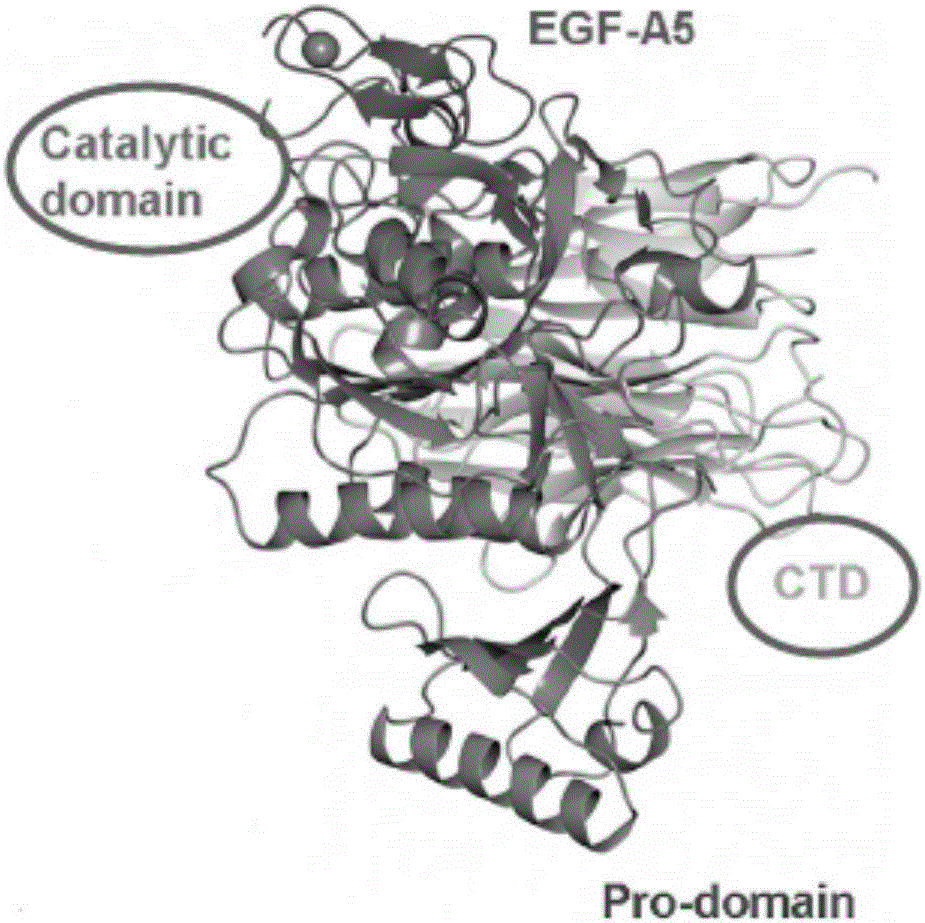
[0046] figure 2 The nucleotide sequences of the catalytic domain and C-terminus of PCSK9 and the amino acid sequences of the corresponding proteins are shown.
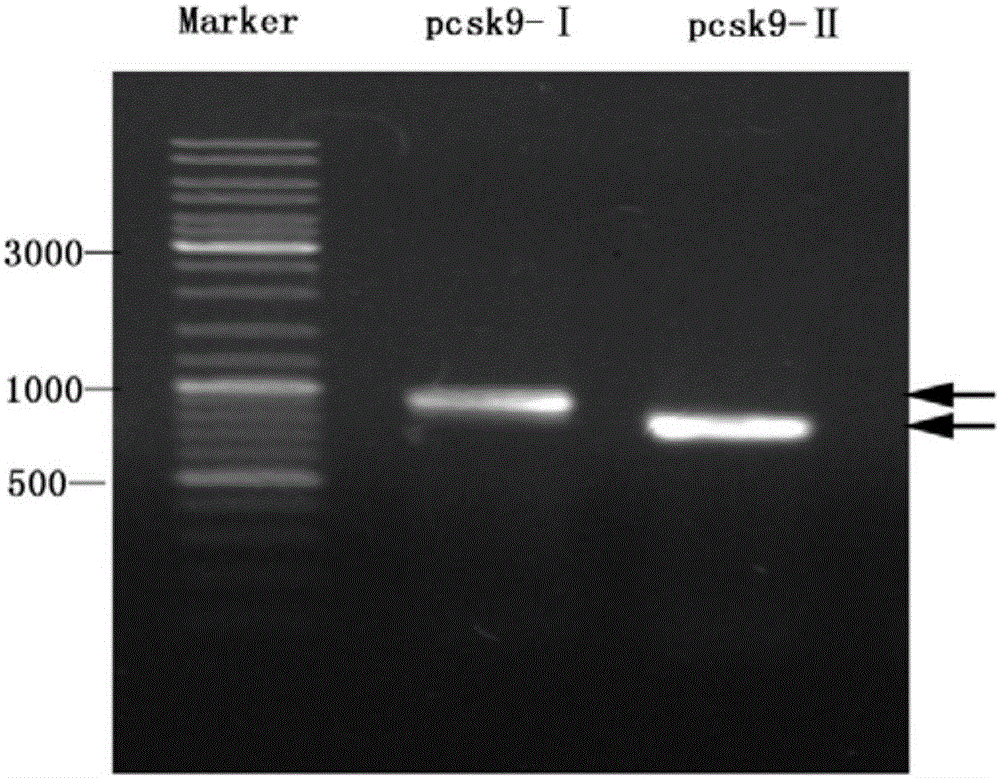
[0047] The catalytic domain (pcsk9-I) and C-terminal (pcsk9-II) cDNA fragments were amplified by PCR reaction using the mouse cDNA sequence as a template, and constructed into an expression vector (pET32a) by endonuclease digestion and DNA ligase ligation Above, they were designated as pET32a-pcsk9-I and pET32a-pcsk9-II, respectively.
[0048] The construction of the pET32a-pcsk9-I vector is as follows:
[0049] Using the cDNA of mouse pcsk9 as a template, the corresponding primers were designed and synthesized.
[0050] Upstream: GG GGTACC AGCATCCCATGGAACCTGGA (SEQ No. 7); downstream: CGG TCA ATGGGTGCTGGGGGGC AGTG (SEQ No. 8). The italic part is the EK enzyme site, the underlined part is the KpnI restricti...
Embodiment 2
[0056] Example 2 Expression and purification of different structural domain PCSK9-I and PCSK9-II proteins
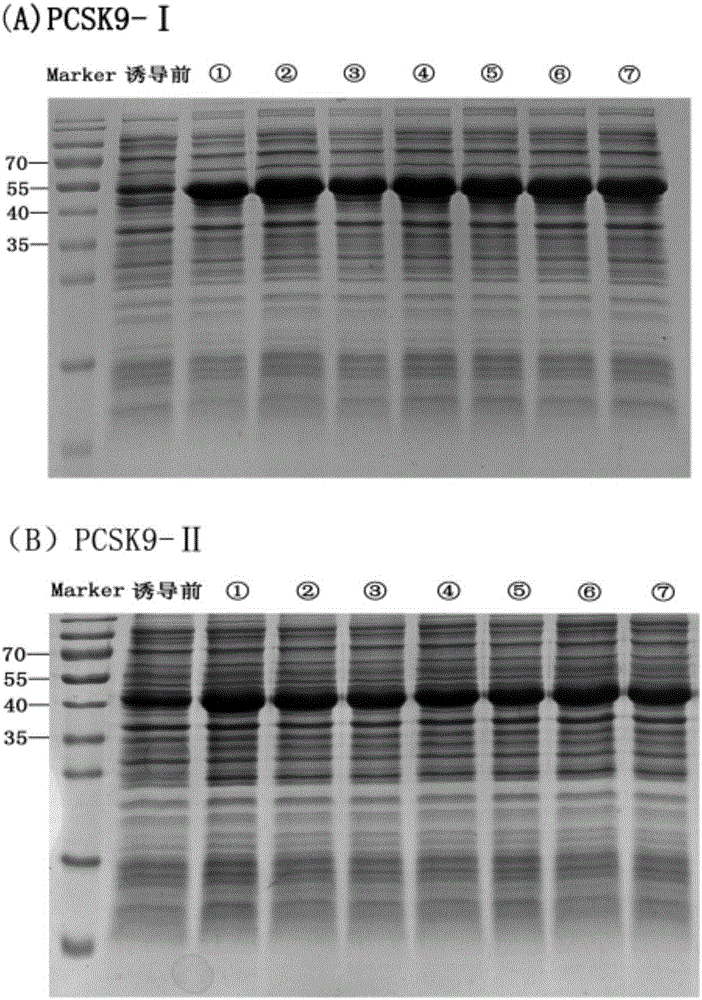
[0057] The recombinant expression plasmid was transformed into Escherichia coli strain BL21(DE3) for expression, and the expression was induced by 0.01M IPIG at 25°C and 220rpm for 8h, and the strains with high expression of PCSK9-I and PCSK9-II proteins were screened out, and the results of electrophoresis showed that the obtained The size of the target protein was similar to the expected ( image 3 ). Soluble identification of the recombinant protein mainly exists in the form of inclusion bodies. The inclusion body protein was washed, refolded and ion-exchange chromatography to obtain relatively pure PCSK9-Ⅰ and PCSK9-Ⅱ recombinant proteins, which were correctly identified by immunoblotting ( Figure 4 ), and the yield can reach 1-2mg / ml.
[0058] The experimental results showed that PCSK9-I and PCSK9-II recombinant proteins were expressed in Escherichia coli strain...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com