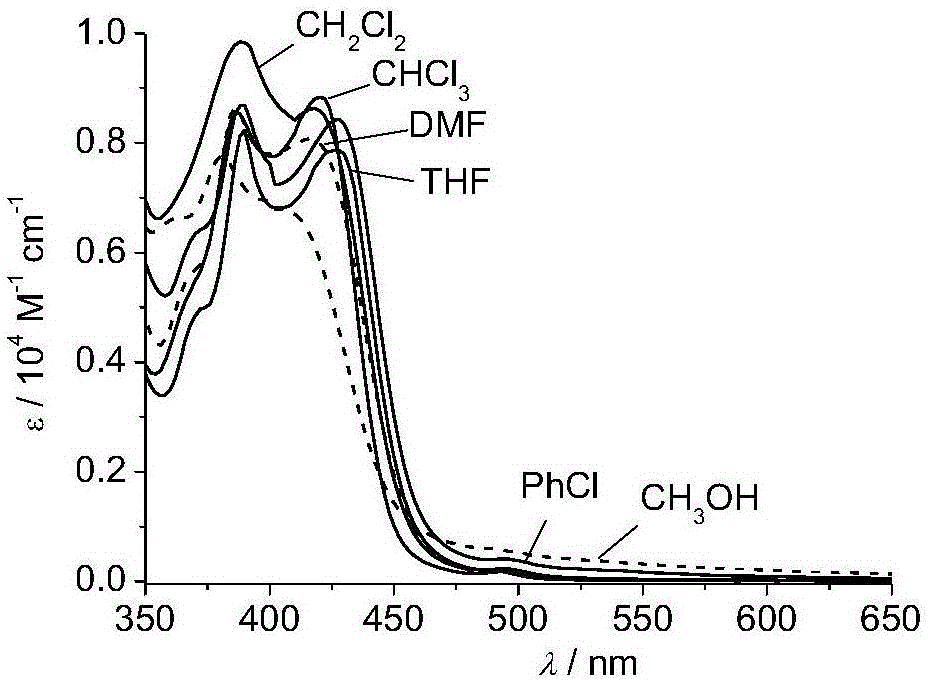
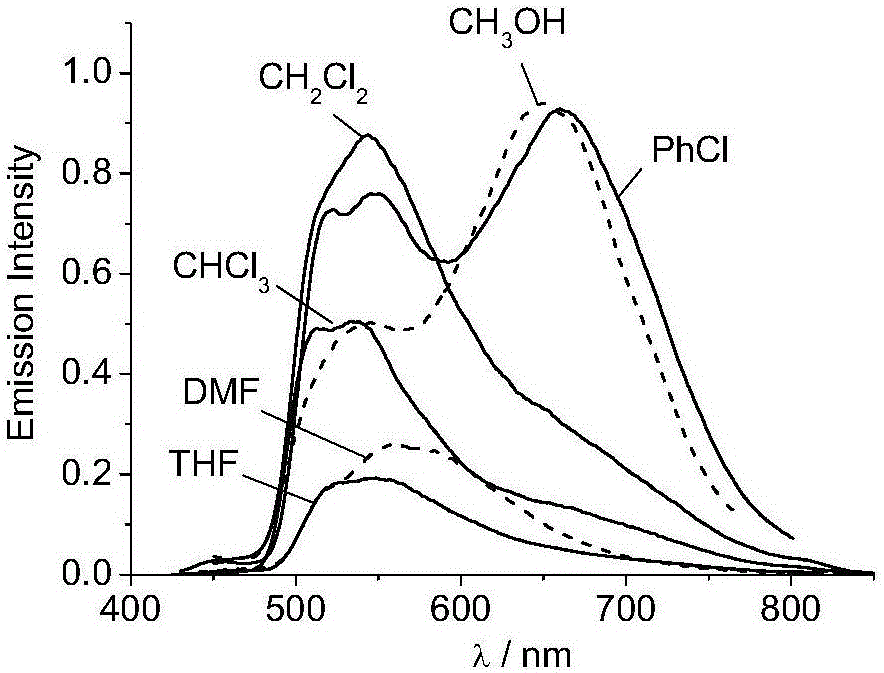
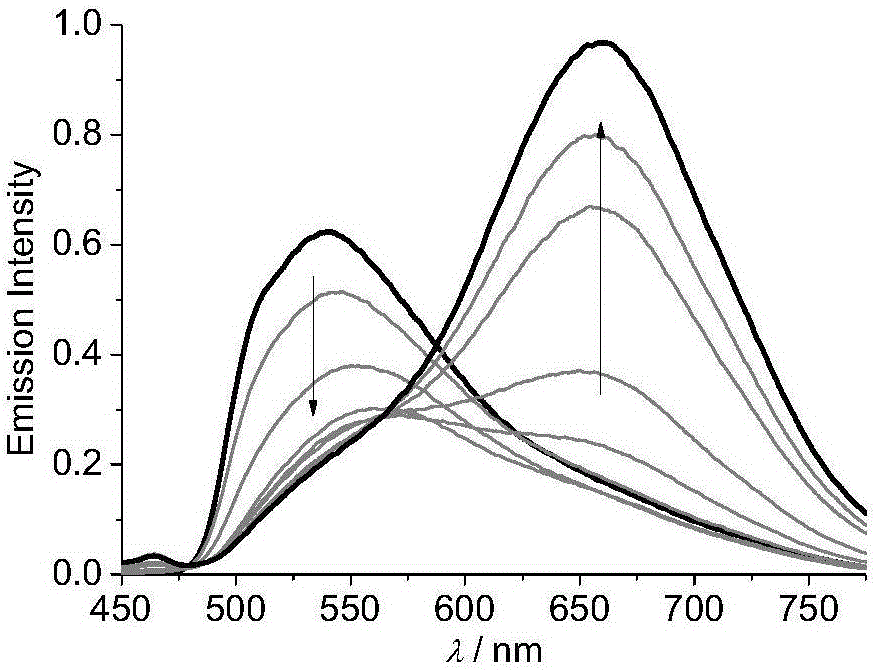
U-shaped dicaryon metal platinum complex with polymorphic light emission and preparation method of U-shaped dicaryon metal platinum complex
A dual-nuclear metal, platinum complex technology, applied in platinum group organic compounds, platinum group organic compounds, luminescent materials, etc., can solve problems such as low quantum yield, and achieve the effect of improving polymorphic species
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] The synthesis of embodiment 1 binuclear metal platinum complex 1-1
[0051] 1) Preparation of 3,5-dipyridineaniline: The synthesis of 3,5-dipyridineaniline refers to Gong Z-L, Zhong Y-W. Stepwise Coordination Followed by Oxidation Mechanism for the Multichannel Detection of Cu 2+ in an Aqueous Environment[J].Organometallics,2013,32:7495-7502.
[0052]
[0053] 2) Preparation of 1,3-bis(3,5-bis(pyridin-2-yl)phenyl)urea: 3,5-dipyridineaniline (123mg, 0.5mmol), triphosgene (150mg, 0.5mmol) ) and 8.0 mL of dichloromethane solvent were placed in an ice bath, and then 0.3 mL of triethylamine was added dropwise for about 2 minutes. After the dropwise addition is completed, keep warm and continue to react for 1.0 hour, then continue to add 4.0 mL of pyridine and 3,5-dipyridine aniline (123 mg, 0.5 mmol), then heat to reflux for 3 hours, TLC detection, after the reaction is completed, stop the reaction and cool to room temperature. The solvent was removed by rotary evapora...
Embodiment 2
[0071] The synthesis of embodiment 2 binuclear metal platinum complexes 1-2
[0072] 1) Preparation of 1,3-bis(3,5-bis(pyridin-2-yl)phenyl)-1,3-dipropylurea: Dilute 40.0 mg of NaH (60%, 1.0 mmol) with n-hexane (5mL×3) was washed and dissolved in 3mL DMF solvent, and gradually added to the compound containing compound 1,3-bis(3,5-bis(pyridin-2-yl)phenyl)urea (130mg, 0.25mmol) 5mL of DMF solution, add part C dropwise after each addition 3 h 7 I (total 128 mg, 0.75 mmol). After the dropwise addition was completed, continue stirring at room temperature for 4 hours, and TLC detection. After the reaction was completed, 30 mL of dichloromethane was added to dissolve after the solvent was removed by rotary evaporation under reduced pressure, and the organic layer was washed with water (30 mL × 3), K 2 CO 3 Dry, remove the solvent by rotary evaporation under reduced pressure, separate and purify by column chromatography (eluent: dichloromethane / ethyl acetate 50:1, v / v) to obtain 12...
Embodiment 3
[0076] The synthesis of embodiment 3 binuclear metal platinum complexes 1-3
[0077] 1) Preparation of 1,3-bis(3,5-bis(pyridin-2-yl)phenyl)-1,3-di-n-hexyl urea: Add 40.0 mg of NaH (60%, 1.0 mmol) to n-hexane After washing with alkane (5mL×3), it was dissolved in 3mL DMF solvent, and gradually added to the solution containing compound 1,3-bis(3,5-bis(pyridin-2-yl)phenyl)urea (130mg, 0.25mmol ) in 5mL of DMF solution, add part C dropwise after each addition 6 h 13 I (total 160 mg, 0.75 mmol). After the dropwise addition was completed, continue to stir at room temperature for 3 hours, and TLC detected that after the reaction was completed, 30 mL of dichloromethane was added to dissolve after the solvent was removed by rotary evaporation under reduced pressure, and the organic layer was washed with water (30 mL × 3), K 2 CO 3 Dry, remove the solvent by rotary evaporation under reduced pressure, separate and purify by column chromatography (eluent: dichloromethane / ethyl acetate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com