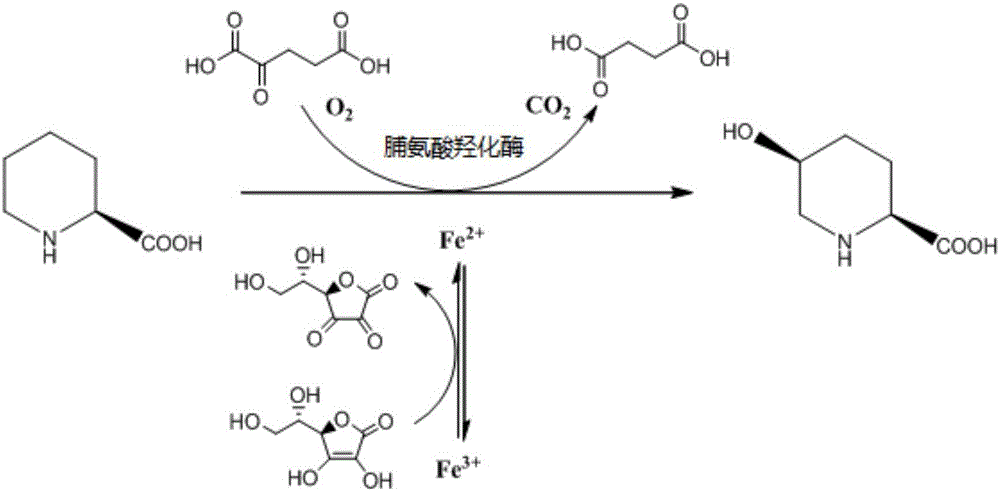
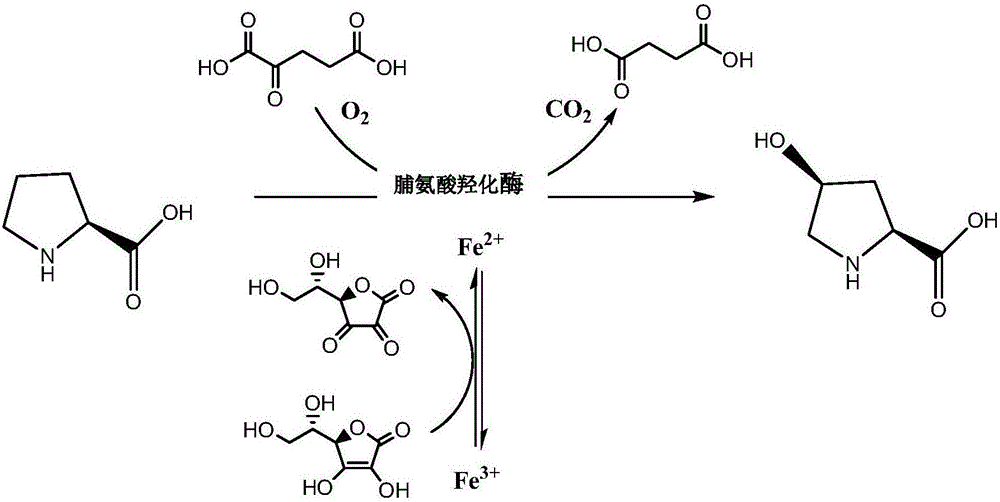
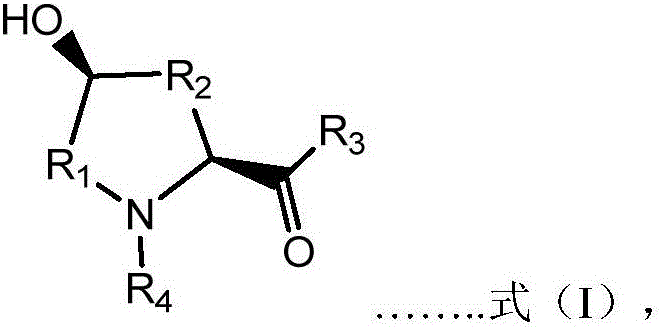
Prolyl hydroxylase and application thereof
A technology for proline hydroxylase and proline hydroxylase activity, which is applied in the fields of genetic engineering and enzyme engineering, and can solve problems such as poor selectivity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0082] Example 1: Recombinant expression of proline hydroxylase
[0083] Codon optimization of the DNA coding sequence SEQ ID NO:1 from Kordia jejudonensis annotated as a hypothetical protein (codon improvement based on E. coli codon preference and degeneracy, designed by Suzhou Jinweizhi Biotechnology Co., Ltd. ), the optimized DNA sequence SEQ ID NO: 3 is obtained, and the encoded proline hydroxylase polypeptide sequence is SEQ ID NO: 4. The coding sequence of SEQ ID NO: 3 was ligated into the pET22b(+) expression vector (purchased from Novagen, product number 69744), then transformed into E. coli BL21 (DE3), and coated on LB containing 50 μg / ml ampicillin Incubate overnight at 37°C in a petri dish. Pick the single clone on the above petri dish and activate it in the test tube, then inoculate it in 500ml LB liquid medium containing 50μg / ml ampicillin, and shake culture at 37℃ to OD 600 = 0.6, add IPTG to a final concentration of 1 mM, and induce expression at 25°C. After 16 h...
Embodiment 2
[0084] Example 2: Preparation of variants of proline hydroxylase
[0085] Take the pET22b(+) expression vector containing SEQ ID NO: 3 as a template, and use primers with mutation sites to obtain a complete linear fragment by full plasmid PCR. After the above PCR product is digested with DPnI to remove the mother template, transform Into Escherichia coli BL21 (DE3), spread on an LB petri dish containing 50μg / ml ampicillin, and cultivate overnight at 37°C. Obtain a single clone containing the amino acid sequence of the proline hydroxylase variant, and then determine the mutation site through trial induction and gene sequencing. Finally, the mutant with a single mutation site is obtained, and then the mutation at the single mutation site is used as the mutant parent, and then the whole plasmid PCR is performed again with primers with mutations at other sites to determine the mutation site again.
[0086] After the mutant strain was activated, it was inoculated into 500ml LB liquid m...
Embodiment 3
[0087] Example 3: Activity screening of proline hydroxylase variants
[0088] The activity screening of proline hydroxylase variants with one amino acid residue difference compared with SEQ ID NO: 2 uses the following 10 mL reaction solution for screening, and the 10 mL reaction solution contains: L-pipecolic acid 30g / L , 5~10wt (1wt means 1g proline hydroxylase variant recombinant wet cell is required to transform 1g main raw material.) Recombinant crude enzyme, 37.3g / L α-ketoglutarate, 6.1g / L L-ascorbic acid, 5mM ferrous ammonium sulfate, reaction pH6.5, reaction temperature 10℃, reaction time 40h. At the end of the reaction, 200μL of the reaction system was taken, 200μL of acetonitrile was added, and 3000μL of purified water was added after mixing. The supernatant was collected by centrifugation at 10000rpm for 5min for HPLC to detect the conversion rate. The activity screening results are shown in Table 1 (Table 1 is based on all conversion rates Activity screening results o...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com