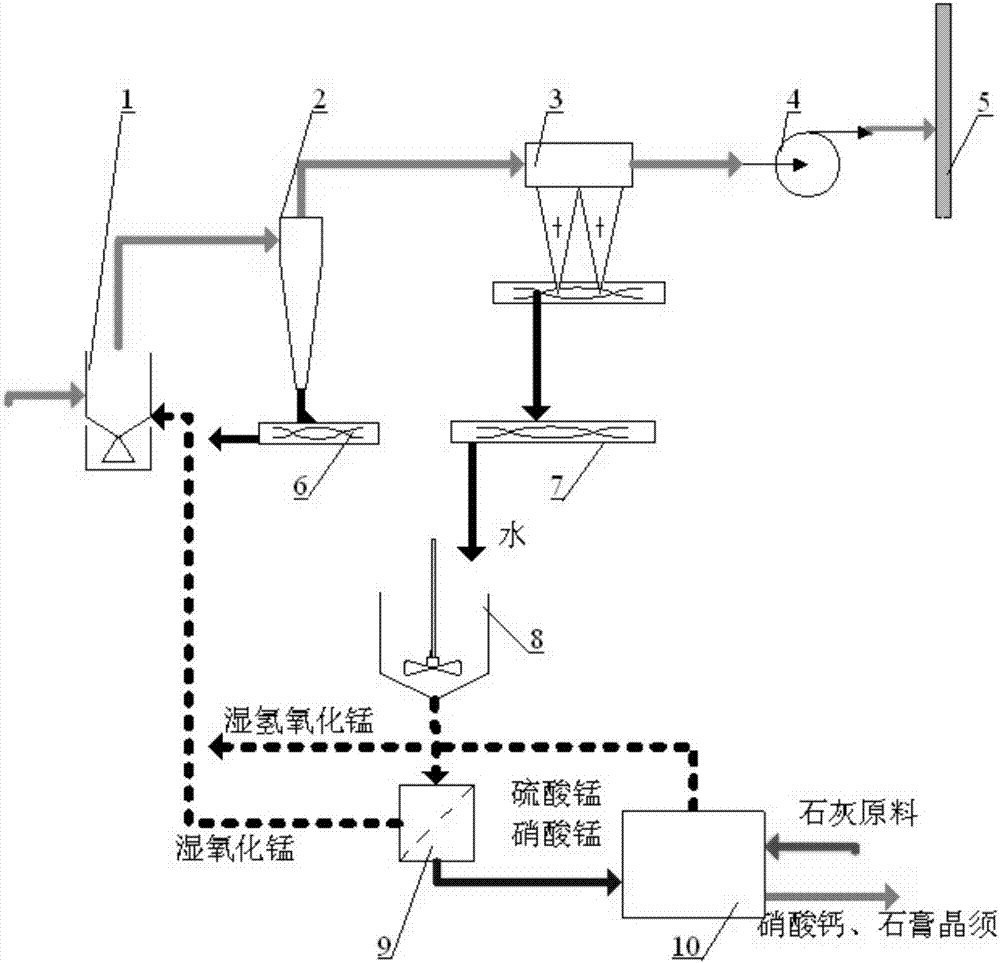
Dry-type deep flue gas desulfurization and denitration integrated method taking manganese hydroxide as circulating work medium
A technology of manganese hydroxide and cyclic work, applied in chemical instruments and methods, separation methods, gas treatment, etc., can solve the problems of potential safety hazards, high cost of desulfurization and denitrification, low resource utilization, etc., and achieve power and heat consumption costs Low cost, improved resource utilization, and convenient cycle operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0064] A dry-type integrated method for flue gas desulfurization and denitrification using manganese hydroxide as a circulating working medium, comprising the following steps:
[0065] 500mL50% manganese nitrate Mn(NO 3 ) 2 The aqueous solution was diluted with water to 2000mL and heated to 85°C, and 150g of Ca(OH) was gradually added to it 2After stirring and reacting for 2 hours, filter and wash, get 350 grams of manganese hydroxide filter cake (water content 60%) and dry at 105°C, grind, put into a 250mL absorption bottle, and the solid height in the bottle is 100mm. Put the solid manganese hydroxide together with the absorption bottle into an oil bath at 110°C-150°C. Pass the preheated simulated flue gas into the absorption bottle at a speed of 7L / min, the composition of the simulated flue gas is: O 2 5%, SO 2 2200mg / m 3 , NO x 502mg / m 3 , the rest is nitrogen N 2 .
[0066] When the adsorption reaction lasted for 10 minutes, the gas outlet temperature of the a...
Embodiment 2
[0072] Take instance 1 and pass into SO 2 29.6g, 100g of manganese oxide with NOx5.7g, dissolved in 350mL water, heated to 85°C, added 32gNaOH in batches, the pH value of the solution was 11, stirred and reacted for 2 hours, vacuum filtered and washed; The cake is dried at 105°C, and the remaining 1 / 3 of the wet filter cake is mixed with the dried part, and put into an absorption bottle with a solid height of 80mm. Put the manganese oxide solid together with the absorption bottle into the oil bath at a speed of 7L / min Pass the preheated simulated flue gas into the absorption bottle, the simulated flue gas composition is: O 2 5.8%, SO 2 2010mg / m 3 , NO x 487mg / m 3 , the rest is nitrogen N 2 .
[0073] When the adsorption reaction lasted for 10 minutes, the gas outlet temperature of the absorption bottle was 140°C, and the outlet gas SO was measured 2 0 mg / m 3 , NO x 46.2 mg / m 3 , the calculated desulfurization efficiency is 100%, and the denitrification efficiency...
Embodiment 3
[0079] Take instance 2 and pass into SO 2 25g, NO x 120g of 4.3g of manganese oxide was dissolved in 300°C of water, heated to 85°C, 80mL of 25% ammonia water was added in batches, the pH of the solution was 10..5, and after stirring for 2 hours, vacuum filtration and washing were performed; 2 / 3 The filter cake is dried at 105°C, and the remaining 1 / 3 of the wet filter cake is mixed with the dried part, and put into an absorption bottle with a solid height of 83mm. Pass the preheated simulated flue gas into the absorption bottle at a high speed, and the simulated flue gas composition is: O 2 6.8%, SO 2 1893mg / m 3 , NO x 472mg / m 3 , the rest is nitrogen N 2 .
[0080] When the adsorption reaction lasted for 10 minutes, the gas outlet temperature of the absorption bottle was 135°C, and the outlet gas SO was measured 2 3mg / m 3 , NO x 53.7mg / m 3 , the calculated desulfurization efficiency is 99.8%, and the denitrification efficiency is 88.6%.
[0081] When the adsorpti...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| specific surface area | aaaaa | aaaaa |
| denitrification rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com