Preparation method of size controllable rhotanium nano particles
A nanoparticle, gold-palladium alloy technology, applied in nanotechnology, metal processing equipment, transportation and packaging, etc., to achieve the effect of simple operation, uniform dispersion and easy control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
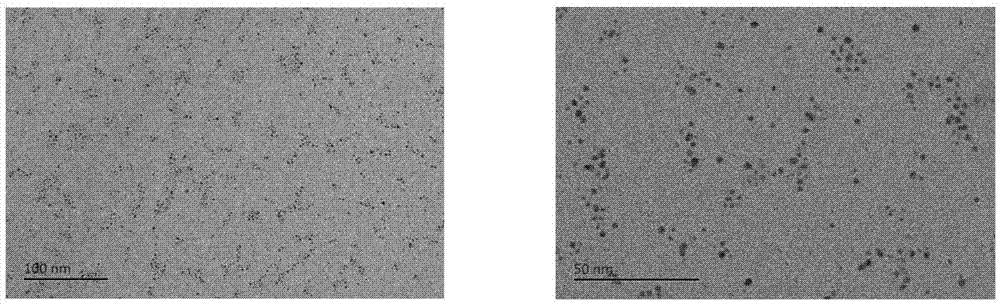
[0024] Synthesis of about 3nm gold-palladium alloy of embodiment 1
[0025] Dissolve the gold organic complex and palladium chloride in tetrahydrofuran, methanol or ethanol solvent, the molar ratio of the gold organic complex to palladium chloride is 1:1, the molar concentration of the gold organic complex in the organic solvent is 3mg / ml, stirred at room temperature for 30 minutes, then added phenethanethiol ligand and stirred for one hour until a large amount of orange precipitate was formed, the molar ratio of the sum of thiol ligand to gold organic complex and palladium chloride is 0.25:1. Finally, an aqueous sodium borohydride solution with a molar ratio of 15:1 to the sum of the gold organic complex and palladium chloride was added to the above reaction, and a black precipitate was formed immediately. After the reaction was carried out for 20 hours, the thiol ligand was washed 4 times with a large amount of methanol, the product was extracted 2 times with dichlorometha...
Embodiment 2
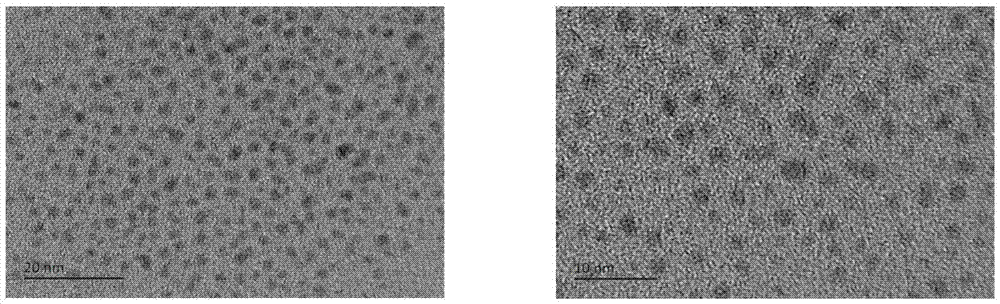
[0027] The synthesis of embodiment 2 2-2.5nm gold-palladium alloy
[0028] Dissolve the gold organic complex and palladium chloride in tetrahydrofuran, methanol or ethanol solvent, the molar ratio of the organic gold source to palladium chloride is 1:1, the molar concentration of the gold organic complex in the organic solvent is 3mg / ml , stirred at room temperature for 30 minutes, then added phenylethyl thiol ligand and stirred for one hour until a large amount of orange precipitate was formed, wherein the molar ratio of thiol ligand to metal source was 0.5:1. Finally, an aqueous sodium borohydride solution with a molar ratio of 15:1 to the sum of the gold organic complex and palladium chloride was added to the above reaction, and a black precipitate was formed immediately. After the reaction was carried out for 20 hours, the thiol ligand was washed 4 times with a large amount of methanol, the product was extracted 2 times with dichloromethane, and the product dissolved in di...
Embodiment 31
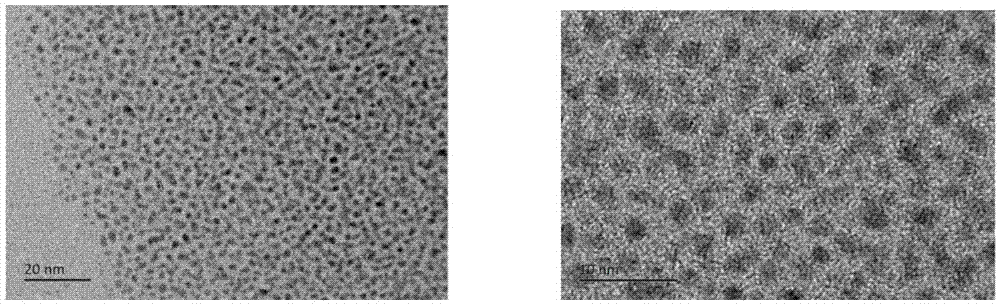
[0030] The synthesis of embodiment 3 1.7-2nm gold-palladium alloy
[0031] Dissolve the gold organic complex and palladium chloride in tetrahydrofuran, methanol or ethanol solvent, the molar ratio of the organic gold source to palladium chloride is 1:1, the molar concentration of the gold organic complex in the organic solvent is 3mg / ml , stirred at room temperature for 30 minutes, then added phenylethyl thiol ligand and stirred for one hour until a large amount of orange precipitate was formed, wherein the molar ratio of thiol ligand to metal source was 1:1. Finally, an aqueous sodium borohydride solution with a molar ratio of 15:1 to the sum of the gold organic complex and palladium chloride was added to the above reaction to immediately generate a black precipitate. After the reaction was carried out for 20 hours, the thiol ligand was washed 4 times with a large amount of methanol, the product was extracted 2 times with dichloromethane, and the product dissolved in dichloro...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
| Size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com