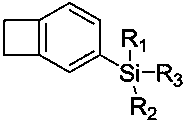
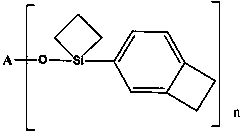
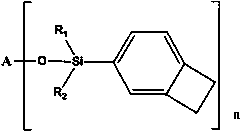
Functionalized organosilicon compound containing benzocyclobutene, and preparation method thereof
A benzocyclobutene functional, organosilicon compound technology, applied in the field of polymer materials, can solve complex problems
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0046] Example 1. Using methylvinylbenzocyclobuten-4-ylchlorosilane and adamantanediol to react to prepare adamantane-containing benzocyclobutene organosilicon compound
[0047] 1,4-adamantanediol (1.682g, 10 mmol), 10ml of tetrahydrofuran, imidazole (1.80g, 26.4mmol), DMAP (0.122g, 1.0 mmol) were added to the three-necked flask, the methyl vinyl benzo Cyclobuten-4-ylchlorosilane (4.593g, 22mmol) and 5ml tetrahydrofuran were added to the constant pressure dropping funnel. Slowly add the mixed solution of methyl vinyl benzocyclobuten-4-yl chlorosilane and tetrahydrofuran to a three-necked flask at room temperature. A white precipitate immediately formed in the reaction solution. After the dropping, the reaction was continued for 2 hours. After the reaction, add ethyl acetate (30ml*3) for extraction, wash the organic layer with deionized water (30ml*2), and dry with anhydrous magnesium sulfate. After filtration, the solvent was removed by rotary evaporation, and purified by dist...
Embodiment 2
[0049] Example 2. Using methyl vinyl BCB chlorosilane to react with 1,4-bis(hydroxydimethylsilyl)benzene to prepare a phenylene-containing structural benzocyclobutene organosilicon compound
[0050] Add 1,4-bis(hydroxydimethylsilyl)benzene (0.6g, 3.5mmol), 10ml tetrahydrofuran, into a three-necked flask, add methylvinylbenzocyclobuten-4-ylchlorosilane (1.4 g, 7mmol), 1.2ml triethylamine, and 5ml tetrahydrofuran were added to the constant pressure dropping funnel. Slowly add methyl vinyl benzocyclobuten-4-yl chlorosilane, triethylamine and tetrahydrofuran mixed solution to the three-necked flask at room temperature. A white precipitate immediately formed in the reaction solution. After the dropping, the reaction was continued for 2 hours. After the reaction, add ethyl acetate (30ml*3) for extraction, wash the organic layer with deionized water (30ml*2), and dry with anhydrous magnesium sulfate. After filtration, the solvent is removed by rotary evaporation, and purified by dist...
Embodiment 3
[0052] Example 3. Using methyl vinyl BCB chlorosilane and 1,4-bis (hydroxydimethylsilyl) biphenyl to prepare biphenylene-containing structural benzocyclobutene organosilicon compound
[0053] Add 1,4-bis(hydroxydimethylsilyl)biphenyl (2.12g, 7mmol), 2.4ml triethylamine, 20ml tetrahydrofuran, into a three-necked flask, add methylvinylbenzocyclobutene-4 -Base chlorosilane (2.8g, 14mmol), 5ml tetrahydrofuran were added to the constant pressure dropping funnel. Slowly add a mixed solution of methyl vinyl benzocyclobuten-4-yl chlorosilane and tetrahydrofuran to a three-necked flask at room temperature. A white precipitate immediately formed in the reaction solution. After the dropping, the reaction was continued for 2 hours. After the reaction, add ethyl acetate (30ml*3) for extraction, wash the organic layer with deionized water (30ml*2), and dry with anhydrous magnesium sulfate. After filtration, the solvent is removed by rotary evaporation, and purified by distillation under re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com