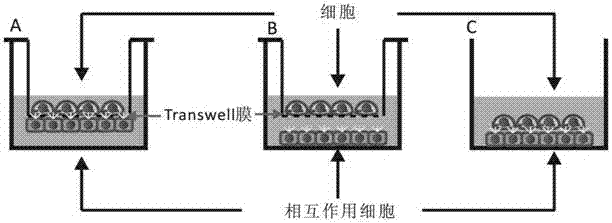
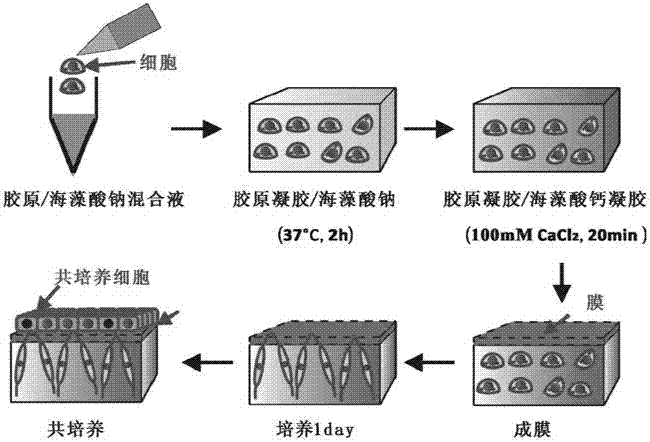
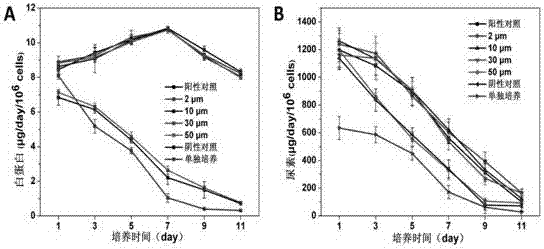
Method for regulating distance between two species of cells
A cell and distance technology, applied in the field of materials and cells, can solve the problem of limited ability to regulate the distance between cells, achieve the effect of continuous controllable film thickness, low cost, and avoid materials and processes
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1: A method for regulating the distance between hepatocytes and fibroblasts
[0025] The 15mg / mL sodium alginate solution and the 3.6mg / ml collagen solution were mixed evenly at a volume ratio of 1:2, so that the final concentrations of the sodium alginate and collagen solutions were 5mg / mL and 2.4mg / mL, respectively. Then mix the human fibroblasts with the mixed sodium alginate and collagen solution, and adjust the cell density to 2×10 6 cells / mL. 300 μl of the cell suspension was slowly added to a circular glass slide with a diameter of 3 cm placed in a cell culture plate, and placed in a cell culture incubator for 2 hours to make the collagen gel. Add 1 mL of 100mM CaCl 2 The solution was added to the cell culture plate, calcified for 20min, sodium alginate and Ca 2+ Chelation forms calcium alginate gel, and finally collagen / calcium alginate gel is obtained. Then, add 1.5mg / mL chitosan (molecular weight 65kDa, degree of deacetylation 90%) solution, by con...
Embodiment 2
[0026] Embodiment 2: A kind of method for regulating the distance between human embryonic stem cells (hES) and fibroblast cells
[0027] First, arginine-glycine-aspartic acid (RGD) modified sodium alginate was prepared. Sodium alginate (molecular weight 430kDa, ratio of guluronic acid to mannuronic acid 1.5) was dissolved in 2-(N-morpholino)ethanesulfonic acid (MES) buffer (pH 6.5) containing 0.5M NaCl ), to obtain a 1% (W / V) sodium alginate solution. Add 1-(3-dimethylaminopropyl)-3-ethylcarbodiimide (EDC), N-hydroxysulfosuccinimide (sulfo-NHS) and RGD polypeptide, and stir at room temperature for 24 hours. The molar ratio of EDC to sodium alginate was 1:20, the molar ratio of EDC to sulfo-NHS was 2:1, and the mass ratio of RGD to sodium alginate was 1:1000. Then dialyzed and freeze-dried to obtain RGD-modified sodium alginate.
[0028] Afterwards, 5 mg of RGD-modified sodium alginate was weighed and dissolved in 100 mL of physiological saline to prepare a sodium alginate s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com