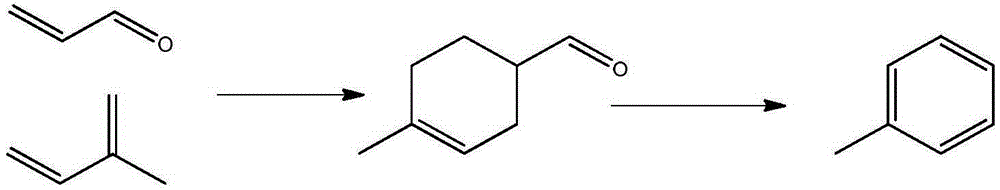
Method for synthesis of toluene from 4-methyl-3-cyclohexene-1-carbaldehyde
A cyclohexene formaldehyde, methyl technology, applied in chemical instruments and methods, hydrocarbons, hydrocarbons, etc., can solve the problems of long process route and high energy consumption, and achieve simple reaction procedures, high selectivity, and high reaction efficiency. short route effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
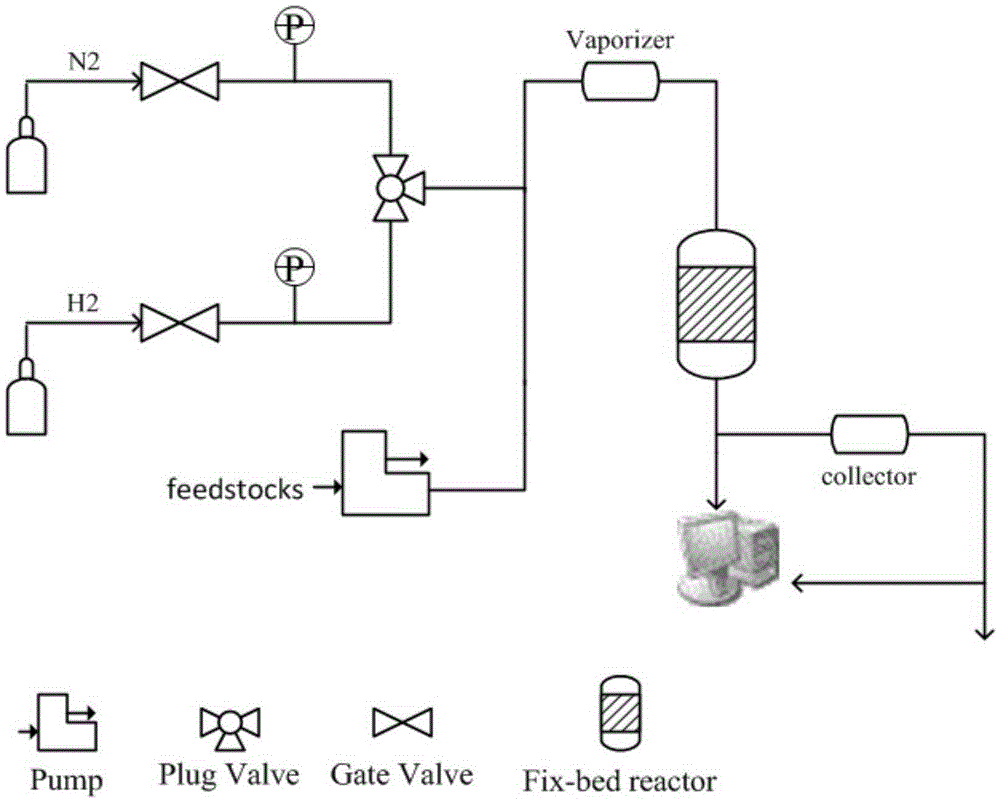
[0030] Preparation of supported catalysts: All supported catalysts were prepared by equal-volume impregnation method, with Pt / Al 2 o 3 As an example, the preparation process is as follows: Weigh 1.59g of chloroplatinic acid solution containing Pt 3.767wt%, add water to dilute to 3.6g, and 5.94g of alumina (ground to 60-80 mesh, specific surface area> 150m 2 / g, pore volume > 0.37m 3 / g, bulk density 710kg / m 3 ) in the solution, baked at 60°C for 4h, at 80°C for 1h, at 120°C for 12h, and calcined at 500°C for 4h in air, and then reduced to room temperature with hydrogen (60ml / min / g) at 300°C. After cooling down to room temperature, O 2 / N 2 Mixed gas (O 2 Volume content 1%) in passivation 4h, obtain 1wt%Pt / Al 2 o 3 .
[0031]According to the above method, Fe, Ru, Ir, Rh, Pd, Ni, etc., which may be supported by activated carbon, alumina, magnesia, silica, zirconia, titania, silica-alumina molecular sieve, phosphorus-aluminum molecular sieve, etc. One or more catalysts, ...
Embodiment 2
[0034] Embodiment 2: (Patent Document 2)
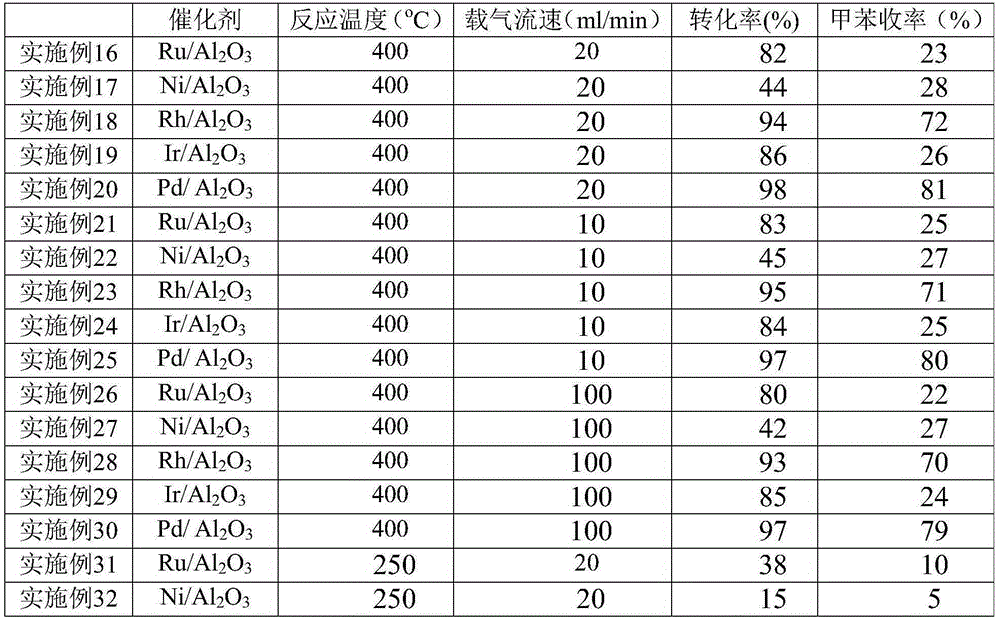
[0035] Add 200 mg of catalyst D (platinum-containing alumina) to a quartz tube with a total length of 300 mm and an inner diameter of 6 mm, heat it to 400 ° C, and circulate nitrogen as a carrier gas from the upper part of the reaction tube at a flow rate of 10 ml / min. 2 To remove moisture for carrier gas purging for 1 hour, the 4-methyl-3-cyclohexene formaldehyde obtained in Example 1 is injected into the vaporization chamber at 1.84g / h, under the flow of nitrogen It was supplied to the catalyst layer, and after reacting for 0.5 h, 767 mg of an organic layer was obtained from the collection vessel at the lower end of the reaction tube. According to GC-MS, the conversion rate of the substrate was 93%, the yield of p-xylene was 41%, and the yield of p-tolualdehyde was 4%. Compared with implementation example 3 (product is mainly toluene), the liquid space velocity of implementation example 2 is 9.2h -1 , the liquid space velocity of ...
Embodiment 3
[0037] 1wt% Pt / Al is filled in the tubular reactor (inner diameter 10mm) 2 o 3 (60-80 mesh) solid catalyst 1.0g, be heated to 400 ℃, with the flow velocity of 20ml / min, circulate as the nitrogen of carrier gas from the top of reaction tube, with N 2 Purging the carrier gas for 1 hour to remove moisture, inject the 4-methyl-3-cyclohexene carboxaldehyde obtained in Example 1 into the vaporization chamber at 1 ml / h, and supply it under the guidance of nitrogen gas To the catalyst layer, after 1 hour of reaction, 724 mg of an organic layer was obtained from the collection vessel at the lower end of the reaction tube. (If the product is completely toluene, only 696 mg in theory). The conversion rate and toluene yield were quantitatively calculated in combination with GC-MS, and the reaction results are listed in Table 1.
PUM
| Property | Measurement | Unit |
|---|---|---|
| specific surface area | aaaaa | aaaaa |
| density | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com