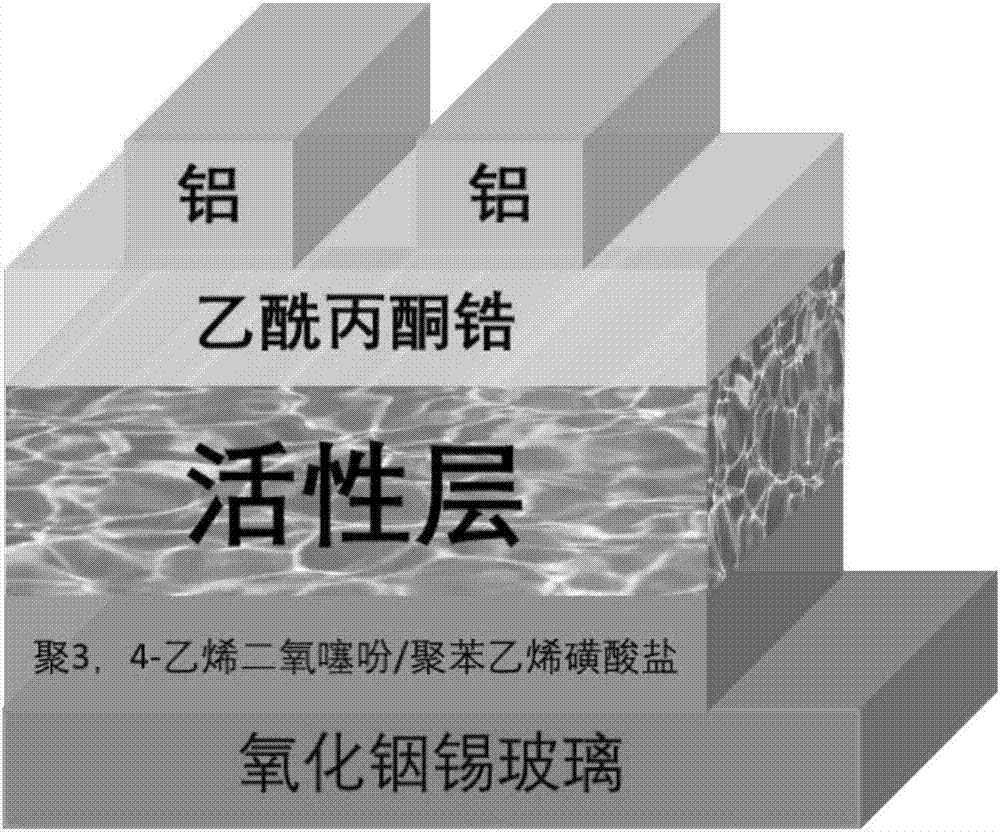
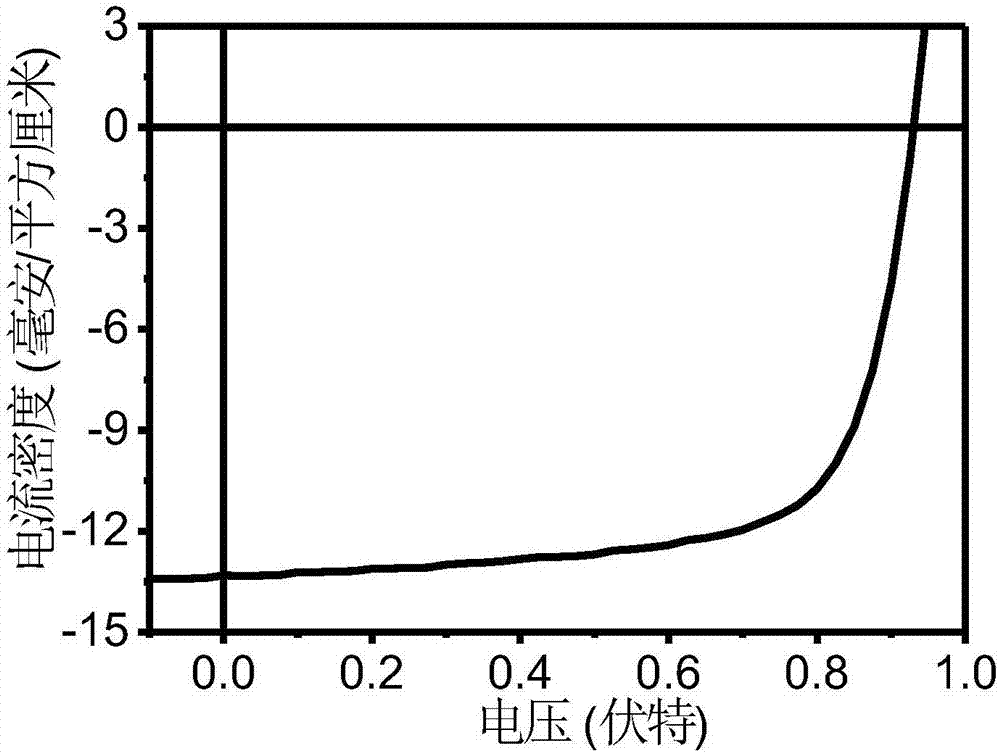
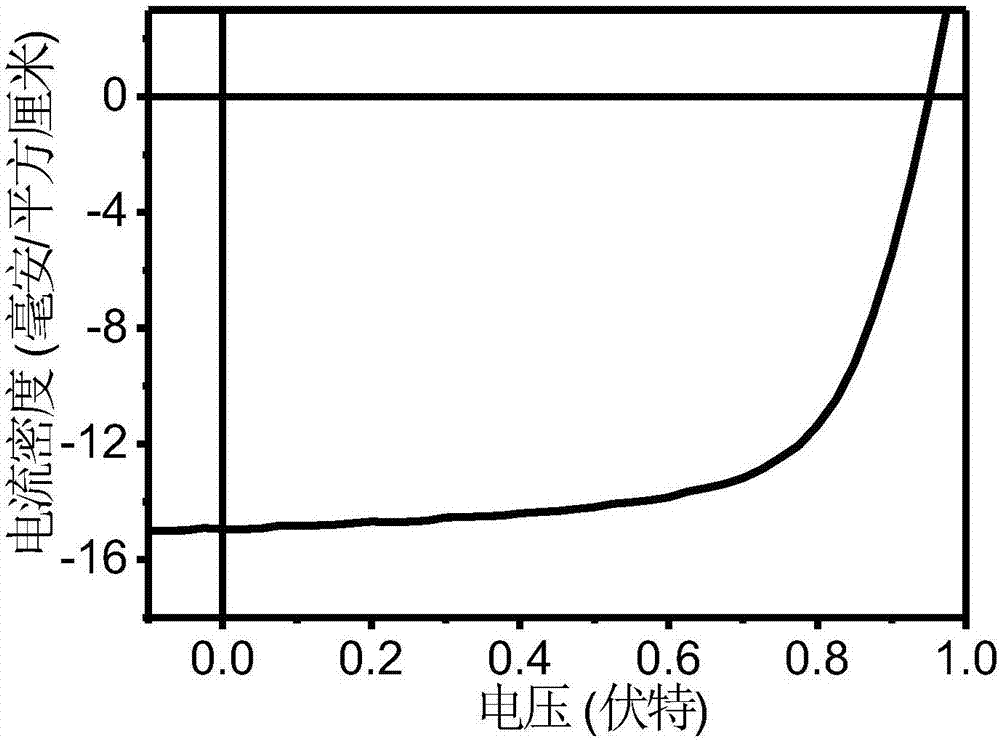
A-D-A conjugated molecule based on thieno cyclopentanedione derivative and preparation method of A-D-A conjugated molecule
A technology of cyclopentadione and conjugated molecules, which is applied in the field of A-D-A conjugated molecules and its preparation, can solve the problems of difficult adjustment of absorption and energy levels, no absorption of visible light, and difficult purification, and achieve high open circuit voltage and fill factor, High photoelectric conversion efficiency and good solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0063] Embodiment 1: the preparation of compound 1
[0064]
[0065] Add compound a (96.3mg, 0.1mmol) and compound d (100mg, 0.5mmol) in a round bottom flask, repeatedly exhaust the gas in the reaction vessel and inject inert gas to get rid of the air in the reaction vessel, then add 10mL of chloroform and stir , add pyridine 0.1mL after completely dissolving; carry out stirring reaction at a temperature of 80°C for 12 hours, then cool to room temperature; the obtained reaction solution is rotary evaporated to remove the solvent, and the obtained solid product is subjected to silica gel (200-300 mesh) column chromatography For separation, the eluent was petroleum ether / dichloromethane (volume ratio 1:1), and the product was a dark blue solid (101 mg, 76%), which was the A-D-A conjugated molecule 1. Theoretical value of element analysis: C, 77.79; H, 5.92; N, 4.22; found value: C, 77.82; H, 5.94; N, 4.25;
Embodiment 2
[0066] Embodiment 2: the preparation of compound 3
[0067] Compound 3 was prepared using the same method as compound 1, except that compound a was replaced by the corresponding thienocyclopentanedione derivative. Elemental analysis theoretical value: C, 78.11; H, 6.26; N, 4.05; found value: C, 78.09; H, 6.24; N, 4.00;
Embodiment 3
[0068] Embodiment 3: the preparation of compound 6
[0069] Compound 6 was prepared using the same method as compound 1, except that compound a was replaced by the corresponding thienocyclopentanedione derivative. Theoretical value of element analysis: C, 77.79; H, 5.92; N, 4.22; found value: C, 77.81; H, 5.94; N, 4.25;
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com