Tipiracil hydrochloride and synthesis method of intermediate 6-(chloromethyl)uracil
A technology of chloromethyluracil and methyluracil, which is applied in the field of synthesis of tipiracil hydrochloride and its intermediate 6-chloromethyluracil, which can solve the problem of high toxicity, unfavorable environmental friendliness, and increase drug safety Hidden dangers and other problems, to achieve the effect of improving yield and purity, increasing environmental friendliness
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
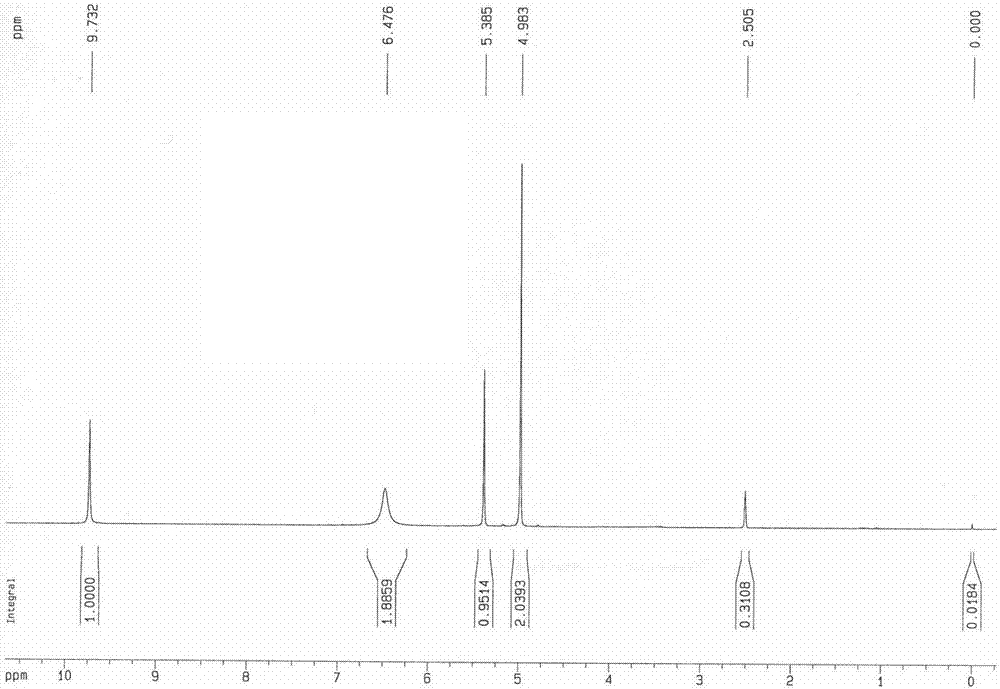
[0039] The invention provides a kind of synthetic method of 6-chloromethyluracil, comprising the following steps:
[0040] oxidizing 6-methyluracil and copper oxide in a solvent to obtain 6-formyluracil;
[0041] Reduction of 6-formyluracil to obtain 6-hydroxymethyluracil;
[0042] Chlorination of 6-hydroxymethyluracil gives 6-chloromethyluracil.
[0043] The synthesis method of 6-chloromethyluracil provided by the present application is environmentally friendly, and the product yield and purity are high, and is more suitable for application in the pharmaceutical industry.
[0044] In the embodiment of the present application, 6-methyluracil, a solvent and copper oxide were mixed in a reaction kettle, and air was introduced into the system at normal pressure, and heated for oxidation reaction to obtain 6-formyluracil.
[0045] The present application uses 6-methyluracil as a raw material and copper oxide as an oxidant to realize the oxidation of methyl in 6-methyluracil to f...
Embodiment 1
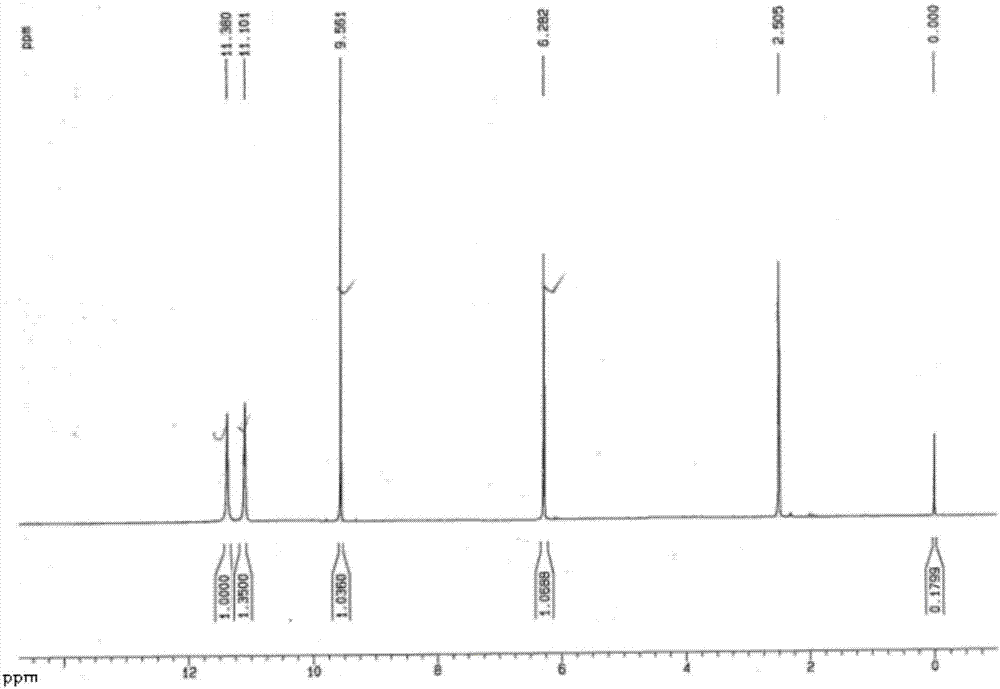
[0081] Synthesis of 6-Formyluracil by Copper Oxide Oxidation
[0082] In a 500L reactor, add 6-methyluracil (20.0kg, 158.59mol) into 150L of acetic acid, add 200g of copper oxide under stirring, and feed air into the system at normal pressure. Heating to 110°C, the color of the reaction solution changed from white turbidity to green turbidity, and the reaction was continued for 5 hours. TLC traced the reaction of the raw material point and disappeared (dichloromethane:methanol=5:1), and the reaction was completed.
[0083] After the reaction was completed, the solvent acetic acid was evaporated under reduced pressure (55°C, 0.09MPa), filtered with diatomaceous earth, the filtrate was cooled to room temperature, adjusted to pH ≈ 1 with concentrated hydrochloric acid, allowed to stand for 8 hours, filtered with suction, and the filter cake was washed with 10L ethanol. Air-dried at 60° C. for 7 hours to obtain light yellow solid compound 1 (8.2 kg, yield 37%).
[0084] The resul...
Embodiment 2
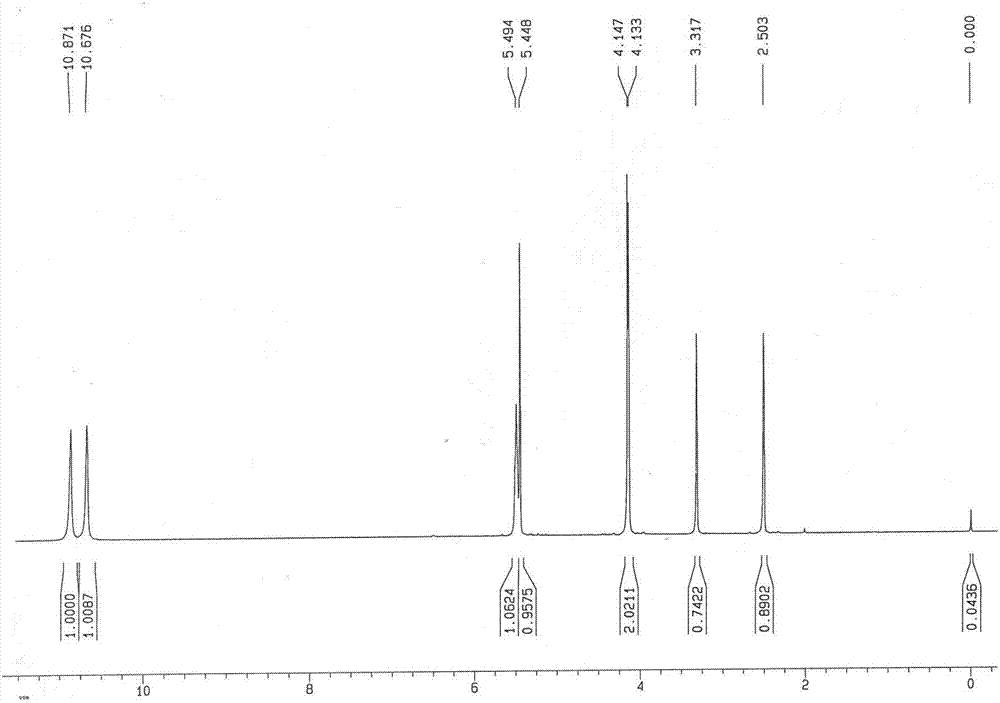
[0095] Synthesis of 6-Hydroxymethyluracil
[0096] In a 500L reactor, add compound 1 (8.2kg, 58.53mol) into 82L tetrahydrofuran, start stirring, add sodium borohydride (2.38kg, 64.38mol), stir at room temperature (20°C) for 20min, and slowly add 100mL Water, there are bubbles, keep the temperature (20°C) and stir for 3 hours, TLC tracking compound 1 point reaction disappears (dichloromethane:methanol=5:1), the reaction is complete.
[0097] Slowly add 42L of water to the reaction solution, evaporate tetrahydrofuran under reduced pressure, the reaction solution is clarified, cooled to room temperature, adjusted to pH ≈ 1 with concentrated hydrochloric acid, left to stand for 3 hours, centrifuged to obtain a filter cake; the filtrate is evaporated to dryness under reduced pressure to obtain a solid, add Stir with 10 L of water for 30 min, filter with suction, combine the filter cakes, and dry under reduced pressure at 60°C for 8 hours to obtain Compound 2 as an off-white solid (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com