Method for animal ubiquitous expression of recombinant human lysozyme
A human lysozyme, systemic technology, applied in the field of obtaining new genes, can solve problems such as inability to achieve physical expression
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
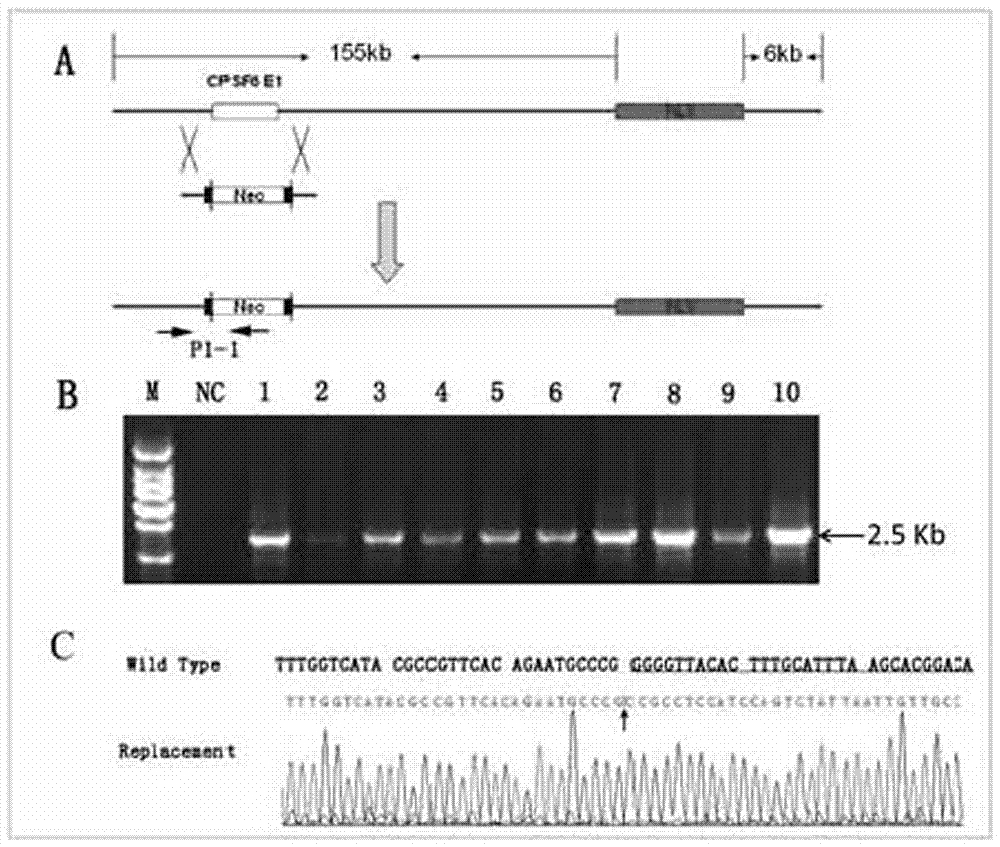
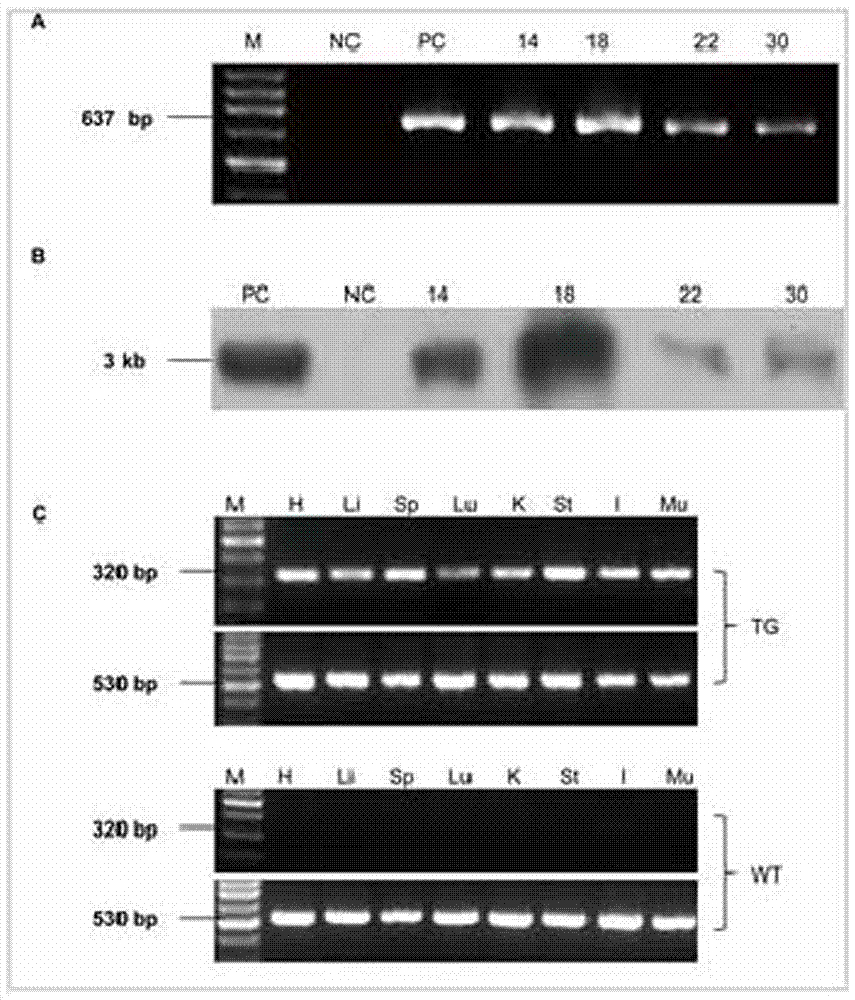
Image
Examples
Embodiment 3
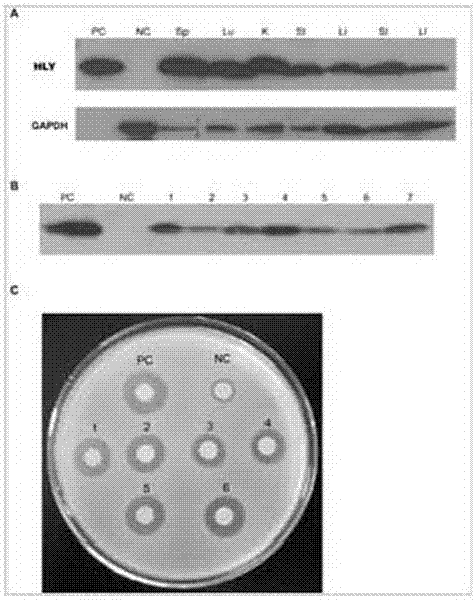
[0080] Example 3: Detection of biological activity of recombinant human lysozyme in serum
[0081] A. Agar plate method
[0082]Take 100uL Micrococcus lyticus respectively ( M. Lysodeikticus ) evenly spread on the 1.5% agar base broth culture plate, and put filter paper sheets with 10uL serum on the culture medium, the serum of ordinary mice is the negative control (NC), and the positive control is the standard product of human lysozyme. PC is 1ug human lysozyme standard. Set up 4 repetitions, culture at 28°C for 36 hours, observe the diameter of the inhibition zone, and visually judge the expression level and activity of recombinant human lysozyme ( image 3 .C).
[0083] The results showed that the serum samples of No. 1-7 F2 generation transgenic mice could all produce obvious inhibition zones, indicating that the recombinant human lysozyme had biological activity.
[0084] , Nephelometric method
[0085] Micrococcus lyticus was cultured in liquid medium for 24 hours,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com