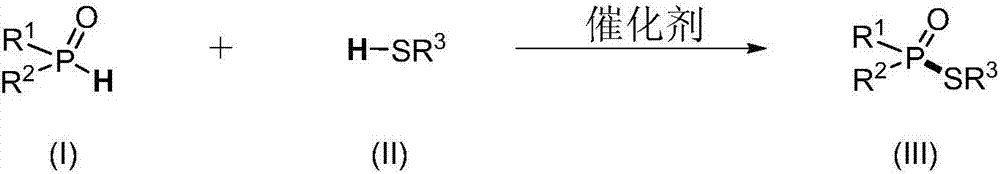
Synthesis method of phosphorothioate compounds and application thereof to synthesis of various medicines
A technology of phosphorothioate and phosphorothioate, which is applied in the field of synthesis of phosphorothioate compounds, can solve the problems of poor functional group compatibility, large substrate limitations, and difficulty in putting into use, and achieve mild and simple reaction conditions, Effect of less waste discharge and lower cost
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0070] Embodiment 1 O, the synthesis of O-diethyl-S-n-octane phosphorothioate
[0071]
[0072] a): Take a 25mL reaction tube, add catalyst cesium carbonate 8.2mg, diethyl phosphite 34.5mg, n-octane mercaptan 43.9mg, acetonitrile 1mL, stir at 40°C for 12 hours, the gas atmosphere is air at a standard atmospheric pressure . After the reaction was completed, 12.7 mg of O, O-diethyl-S-n-octane phosphorothioate was obtained through column chromatography, with a yield of 18%.
[0073] b): Take a 25mL reaction tube, add catalyst cesium carbonate 16.4mg, diethyl phosphite 34.5mg, n-octane mercaptan 43.9mg, acetonitrile 1mL, stir at 40°C for 12 hours, the gas atmosphere is air at a standard atmospheric pressure . After the reaction was completed, 24.0 mg of O, O-diethyl-S-n-octane phosphorothioate was obtained through column chromatography, with a yield of 34%.
[0074] c): Take a 25mL reaction tube, add catalyst cesium carbonate 16.4mg, diethyl phosphite 34.5mg, n-octane mercap...
Embodiment 2
[0103] Example 2 Synthesis of O, O-dibutyl-S-n-octane phosphorothioate
[0104]
[0105] Take a 25mL reaction tube, add catalyst cesium carbonate 41.0mg, dibutyl phosphite 48.6mg, n-octane mercaptan 43.9mg, acetonitrile 1mL, stir at 80°C for 3 hours, the gas atmosphere is oxygen at a standard atmospheric pressure. After the reaction was completed, 77.6 mg of O, O-dibutyl-S-n-octane phosphorothioate was obtained through column chromatography, with a yield of 92%.
[0106] 1 H NMR (400MHz, CDCl 3 ):δ4.16-4.02(m,4H),2.86-2.79(m,2H),1.72-1.65(m,6H),1.47-1.28(m,14H),0.95(t,J=7.2Hz,6H ),0.88(t,J=6.4Hz,3H). 13 C NMR (100MHz, CDCl 3 ):δ67.0(d,J C-P =6.0Hz), 32.0(d, J C-P =7.4Hz), 31.6, 30.7(d, J C-P =4.7Hz), 30.6, 29.0, 28.9, 28.4, 22.5, 18.6, 14.0, 13.5. 31 P NMR (162MHz, CDCl 3 ): δ28.5. HRMS m / z (ESI) calcd for C 16 h 36 o 3 PS[M+H] + 339.2123,found: 339.2118.
Embodiment 3
[0107] Example 3 O, the synthesis of O-diisopropyl-S-n-octane phosphorothioate
[0108]
[0109] Take a 25mL reaction tube, add catalyst cesium carbonate 41.0mg, diisopropyl phosphite 41.6mg, n-octane mercaptan 43.9mg, acetonitrile 1mL, stir at 80°C for 3 hours, the gas atmosphere is a standard atmospheric pressure of oxygen. After the reaction was completed, 63.1 mg of O, O-diisopropyl-S-n-octane phosphorothioate was obtained through column chromatography, with a yield of 82%.
[0110] 1 H NMR (400MHz, CDCl 3 ):δ4.78-4.70(m,2H),2.87-2.79(m,2H),1.72-1.65(m,2H),1.38-1.27(m,22H),0.88(t,J=6.4Hz,3H ). 13 C NMR (100MHz, CDCl 3 ):δ72.3(d,J C-P =6.1Hz),31.7,31.0(d,J C-P =4.7Hz), 30.6(d, J C-P =6.3Hz), 29.1, 28.9, 28.6, 23.8 (d, J C-P =3.3Hz), 23.5(d, J C-P =5.6Hz), 22.6, 14.0. 31 P NMR (162MHz, CDCl 3 ): δ25.8. HRMS m / z (ESI) calcd for C 14 h 32 o 3 PS[M+H] + 311.1810,found: 311.1807.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com