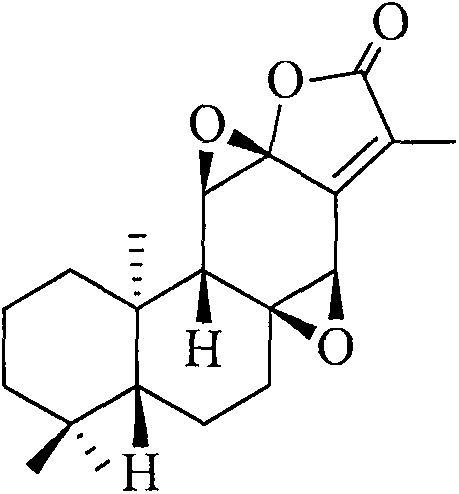
Preparation method of active pharmaceutical ingredient jolkinolide B
A technology of spurge lactone and raw material medicine, applied in the field of natural organic chemistry, can solve the problems of high production cost, complicated operation, low product yield and the like, achieve high yield, improve yield, simple and practical technological process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
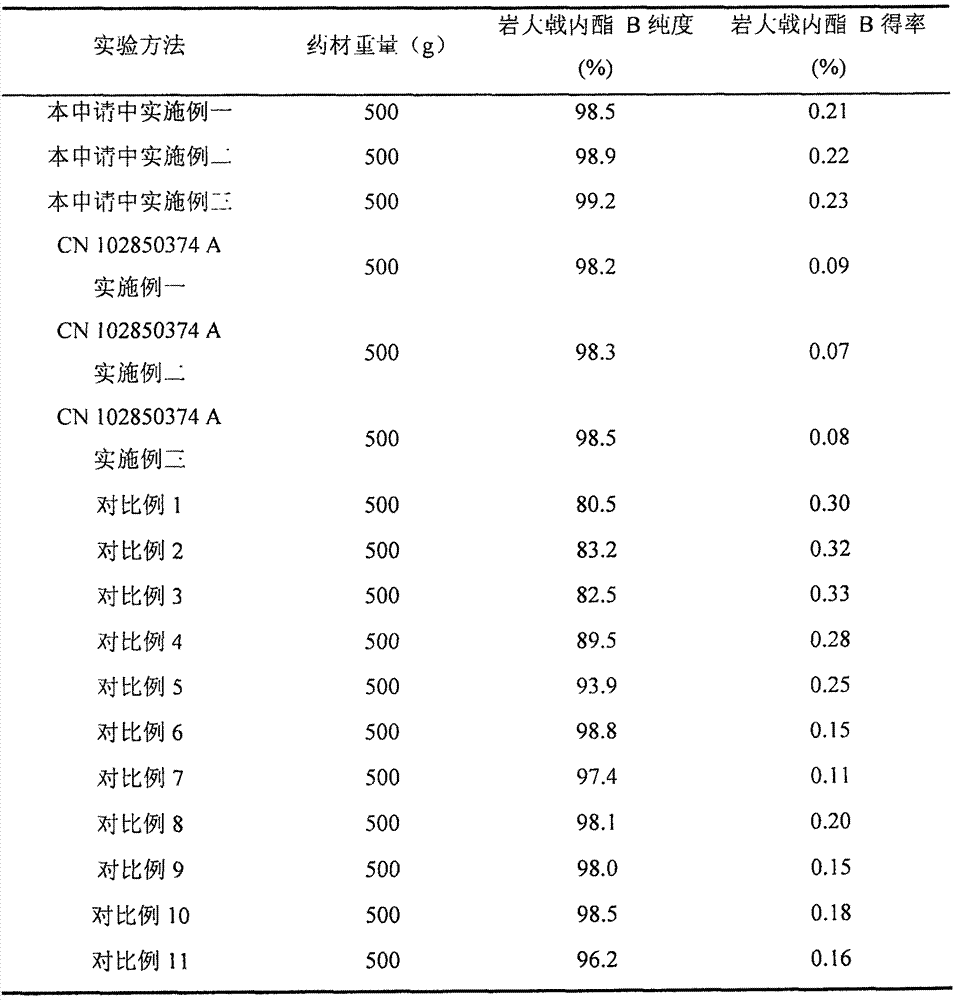
[0042] (1) Grind 500g dried root of Euphorbia chamaejasme into coarse powder, add 6L 60% ethanol for reflux extraction for 3h, filter, add 5L 60% ethanol to the filter residue for reflux extraction for 2h, filter, add 5L 60% ethanol for filter residue Reflux for extraction for 1 hour, filter, combine the extracts, concentrate under reduced pressure until there is no alcohol smell, and the density is 1.11 to obtain a concentrated solution (about 76 g);
[0043] (2) After the concentrated solution was dispersed with a small amount of water, it was extracted 4 times with 2 times the volume of ethyl acetate, the ethyl acetate phase was combined, and the ethyl acetate was recovered to obtain extract I (about 6.5 g);
[0044] (3) After dissolving the extract I with 20% ethanol, put it on the AB-8 macroporous adsorption resin column, pack the column by wet method (the ratio of sample volume to column resin volume is 1:3), and first elute with 20% ethanol 5BV, discarded; 8BV was elute...
Embodiment 2
[0047] (1) Grind 500g dried root of Euphorbia chamaejasme into coarse powder, add 6L 80% ethanol for reflux extraction for 3h, filter, add 5L80% ethanol to the filter residue for reflux extraction for 2h, filter, add 5L 80% ethanol for reflux extraction After 1 hour, filter, combine the extracts, concentrate under reduced pressure until there is no alcohol smell, and the density is 1.12 to obtain a concentrated solution (about 80 g).
[0048] (2) After the concentrated solution was dispersed with a small amount of water, it was extracted 4 times with 2.5 times the volume of ethyl acetate, the ethyl acetate phase was combined, and the ethyl acetate was recovered to obtain extract I (about 5.5 g);
[0049] (3) After dissolving the extract I with 30% ethanol, put it on the D101 macroporous adsorption resin column, and wet-pack the column (the ratio of the sample volume to the column resin volume is 1: 3), and first elute 5BV with 30% ethanol, Discard; then elute 8BV with 85% etha...
Embodiment 3
[0052] (1) Grind 500g dried root of Euphorbia chamaejasme into coarse powder, add 6L 95% ethanol for reflux extraction for 3h, filter, add 5L 95% ethanol to the filter residue for reflux extraction for 2h, filter, add 5L 95% ethanol for filter residue Reflux for extraction for 1 hour, filter, combine the extracts, concentrate under reduced pressure until there is no alcohol smell, and the density is 1.12 to obtain a concentrated solution (about 85 g).
[0053] (2) After the concentrated solution was dispersed with a small amount of water, it was extracted 4 times with 3 times the volume of ethyl acetate, the ethyl acetate phase was combined, and the ethyl acetate was recovered to obtain extract I (about 7.0 g);
[0054] (3) After dissolving the extract I with 40% ethanol, put it on the ADS-17 macroporous adsorption resin column, pack the column by wet method (the ratio of sample volume to column resin volume is 1:3), and first elute with 40% ethanol 5BV, discarded; then 8BV wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com