Site-directed single-substituted pegylated exendin analogue and preparation method thereof
A technology of PEGylation and analogues, which is applied in the field of fixed-point monosubstituted PEGylation of Exendin analogues, which can solve the problems of unclear efficacy and toxicity, adverse effects on stability, easy oxidation of cysteine, etc. , to avoid multiple substitution side reactions, reduce production costs, and avoid side effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
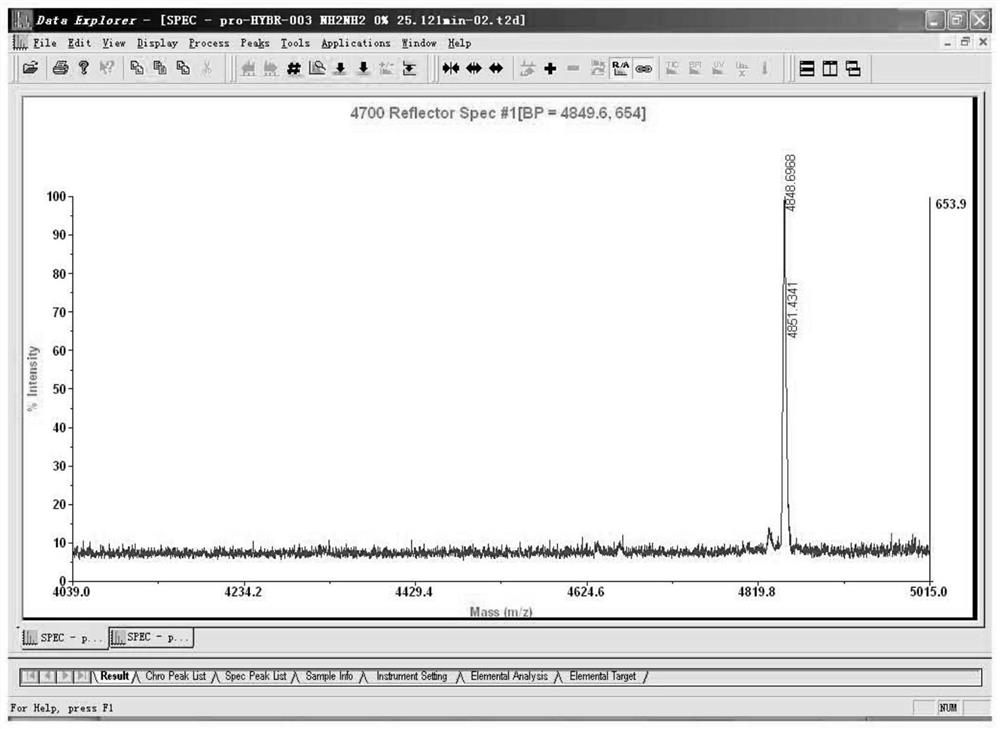
[0066](1) Amino acid monomer used to synthesize
[0067]FMoc-His (TRT) -OH, DDE-His (TRT) -OH, Fmoc-ala-OH, Fmoc-Gly-OH, Fmoc-Glu (OtBu) -OH, Fmoc-Thr (TBU) -OH, FMOC- PHE-OH, FMOC-SER (TBU) -OH, FMoc-ASP (OTBU) -OH, Fmoc-Leu-OH, Fmoc-Lys (Boc) -OH, Fmoc-Lys (DDE) -OH, FMOC-GLN ( TRT) -OH, Fmoc-Val-OH, FMOC-ILE-OH, FMOC-TRP (Boc) -OH, Fmoc-Asn (TRT) -OH, Fmoc-Pro-OH, Fmoc-Cys (TRT) -OH
[0068]Abbreviations are: FmoC 9-fluorenyl methoxycarbonyl; BoC tert-butoxycarbonyl; TRT triphenyl group; OTBU tert-butyloxy; TBU t-butyl; DDE N- [1- (4, 4-dimethyl) Base-2,6-dioxide subunisyl)
[0069](2) reagent: N, N-diisopropylamine, diisopropyl carbon diimide (DIC), N, N-dimethylformamide (DMF), dichloromethane, hexapyridine, 1 - Hydroxybenzotriazole, RINK Amide resin, ninluol, methanol, triisopropylsilane, trifluoroacetic acid.
[0070](3) operation
[0071]A synthesis: Take 0.25 mmol size as an example, weigh 0.5 g of Rink Amide resin is placed in the reactor, and the amino acid is given 1 mmol, activated by...
Embodiment 2
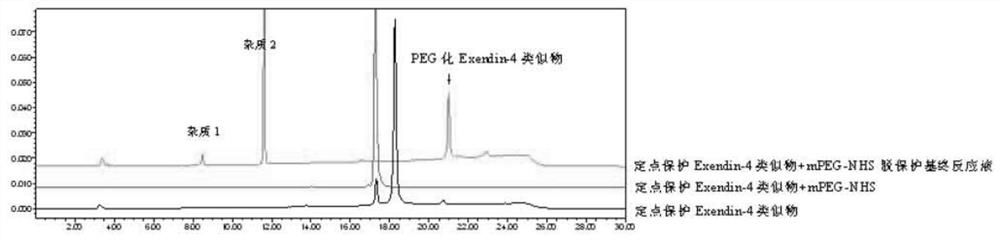
[0089]Method for protecting Exendin-4 analog polyethylene glycolization
[0090]This example uses conventional amino polyethylene glycolification reagents such as (SC-PEG, SS-PEG, NHS-PEG, etc.) to protect the side chain of polyethylene glycolinated reactions on the exendin-4 analog. The amino group is combined with modification. Among them, polyethylene glycolified reagents are preferably 40 kD Y-type NHS-PEG, and preferentially protect exendin-4 analogues.
[0091](FMOC) His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser- (DDE) Lys-Gln-Z-Glu-Glu-Glu-ala-Val-Lys-Leu-Phe- ILE-GLU-TRP-Leu- (DDE) Lys-asn-Gly-Gly-Pro-Ser-Ser-Gly-Ala-Pro-Pro-Pro-Ser- (DDE) Lys or
[0092](FMOC) His-Gly-Glu-Gly-Thr-Phe-Thr-Ser-Asp-Leu-Ser- (DDE) Lys-Gln-Z-Glu-Glu-Glu-ala-Val- (DDE) Lys-Leu -Phe-ILE-GLU-TRP-LEU- (DDE) Lys-asn-gly-gly-pro-ser-ser-gly-ala-prop-pro-pro-ser-lys.
[0093]The point protects the exendin-4 analog 2g, 40 kD Y-type NHS-PEG 26g (with polypeptide molar ratio 1.5: 1) dissolved in 400 ml DMSO, 40 ° C to ...
Embodiment 3
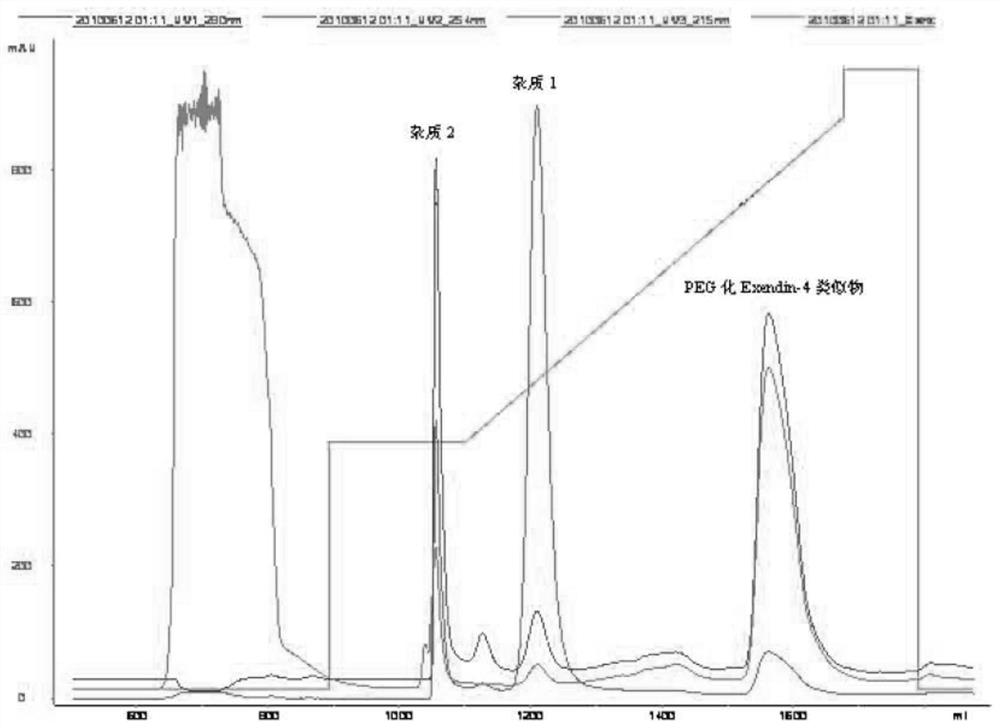
[0096]Polyethylene glycolified Exendin-4 analog isolation purification method
[0097]The fixed-point protection Exendin-4-analog polyethylene glycolized end reaction liquid is respectively obtained by reverse phase HPLC, ion exchange, ultrafiltration, molecular sieve and freeze-drying to obtain pure polyethylene glycolization Exendin-4 analog producers.
[0098]A, Source reverse phase HPLC purification
[0099]Mobile phase: a phase A phase 20mm Hac 5% acetonitrile; B phase 20mm Hac 50% acetonitrile
[0100]Column: GE FineLine Pilot 35
[0101]Filler: Source 30RPC 175ml
[0102]Flow speed: 30ml / min
[0103]Elution gradient: balance 2 column volume, 0-30% 5 min, 30% -100% 5 min
[0104]image 3 It is a polyethylene glycolified Exendin-4 analog source Source purified chromatogram.
[0105]B, SP cation exchange purification
[0106]Mobile phase: a phase 10mm pH 3.5CBS
[0107]B1 phase 10mm pH 3.5cbs + 0.15m NaCl
[0108]B2 phase 10mm pH 3.5CBS + 1.5M NaCl
[0109]Column: GE XK 50
[0110]Filler: SP Sepharose Fast Flow 600ml
[0...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com