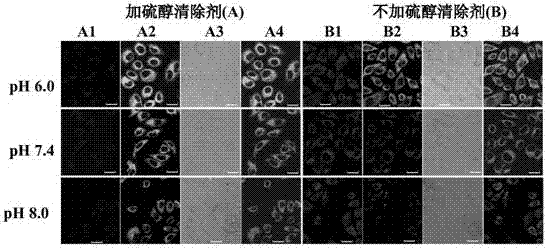
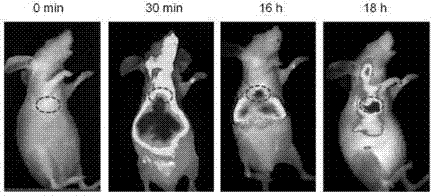
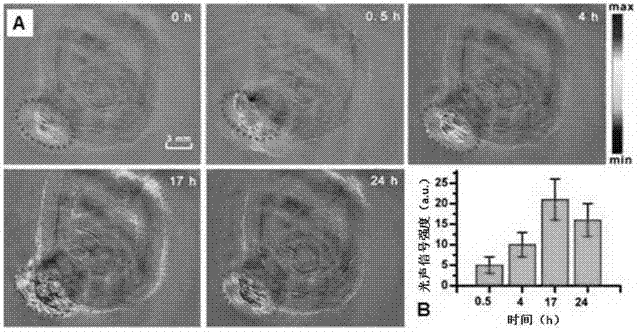
Multifunctional near infrared fluorescent probe and preparation method and application thereof
A fluorescent probe and near-infrared technology, applied in the field of multifunctional near-infrared fluorescent probes and their preparation, to achieve the effects of less background interference, elimination of interference and low toxicity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0089] A method for preparing a multifunctional near-infrared fluorescent probe, comprising the following steps:
[0090] (1) The first compound S 1 Synthesis:
[0091] S 1 The synthetic route of:
[0092]
[0093] S 1 The synthesis steps are as follows:
[0094] At room temperature, add cystamine dihydrochloride (162mg, 0.72mmol), 1.5mL methanol, 416μL triethylamine (3mmol) into a 25mL round bottom flask, then add 2mL acetonitrile, and stir rapidly for about 0.5 hours, the solution becomes transparent , cystamine dihydrochloride is completely dissolved to obtain the first mixed solution;
[0095] then put (abbreviated as Cy.7.Cl, 383mg, 0.6mmol) was dissolved in 4mL of acetonitrile to obtain the second mixed solution, which was then added dropwise to the above-mentioned first mixed solution with a dropping funnel at a rate of about 1 drop / 20s, heat, and react at 35°C for 4 hours under the protection of nitrogen. After the reaction is completed, the reaction solut...
Embodiment 2
[0111] A method for preparing a multifunctional near-infrared fluorescent probe, comprising the following steps:
[0112] (1) The first compound S 1 Synthesis:
[0113] S 1 The synthesis steps are as follows:
[0114]At room temperature, add cystamine dihydrochloride (135mg, 0.6mmol), 1.5mL methanol, 166.4μL triethylamine (1.2mmol) into a 25mL round bottom flask, then add 2mL acetonitrile, and stir rapidly for about 0.5 hours, the solution Become transparent, cystamine dihydrochloride all dissolves, obtains the first mixed solution;
[0115] then put (abbreviated as Cy.7.Cl, 383.1mg, 0.6mmol) was dissolved in 4mL of acetonitrile to obtain the second mixed solution, which was then added dropwise to the above-mentioned first mixed solution with a dropping funnel at a rate of about 1 drop / 20s, heat, and react at 30°C for 6 hours under the protection of nitrogen. After the reaction is completed, the reaction solution after the reaction is rotary evaporated to remove the sol...
Embodiment 3
[0124] A method for preparing a multifunctional near-infrared fluorescent probe, comprising the following steps:
[0125] (1) The first compound S 1 Synthesis:
[0126] S 1 The synthesis steps are as follows:
[0127] At room temperature, add cystamine dihydrochloride (135mg, 0.6mmol), 1.5mL methanol, 332.8μL triethylamine (2.4mmol) into a 25mL round bottom flask, then add 2mL acetonitrile, and stir rapidly for about 0.5 hours, the solution Become transparent, cystamine dihydrochloride all dissolves, obtains the first mixed solution;
[0128] then put (abbreviated as Cy.7.Cl, 383.1mg, 0.6mmol) was dissolved in 4mL of acetonitrile to obtain the second mixed solution, which was then added dropwise to the above-mentioned first mixed solution with a dropping funnel at a rate of about 1 drop / 20s, heat, and react at 40°C for 5 hours under the protection of nitrogen. After the reaction is completed, the reaction solution after the reaction is rotary evaporated to remove the so...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com